Page 177 - Basic physical chemistry for the atmospheric sciences
P. 177
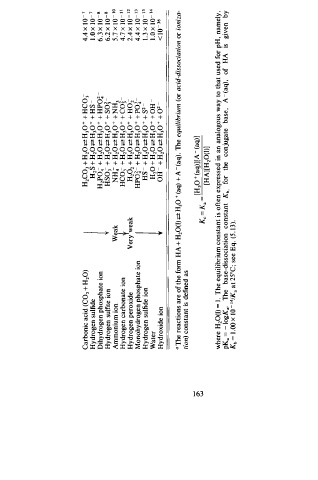
Carbonic acid (C02 + H20) H2C03 + H2 0 <=! H 30 + + HC03 4.4 x 1 0 - 1
Hydrogen sulfide H2S + H20 <=! H 30 + + HS I . O x 1 0 - 1
Dihydrogen phosphate ion 1 H2Po4- + H20 <=! H 30 + + HPO� 6 . 3 x 1 0 - s
Hydrogen sulfite ion HS03 + H 20 <=! H30 + + s o� 6.2 x 1 0 - s
Ammonium ion Weak NH4- + H 20 <=! H 30 + + N H3 5 . 7 x 1 0 - 1 0
Hydrogen carbonate ion HC03 + H 20 <=! H30 + + c o� 4.7 x 1 0 - 1 1
Hydrogen peroxide VerJweak H202 + H2 0 <=! H 30+ + H02 2.4 x 10 - 1 2
Monohydrogen phosphate ion HP o � - + H20 <=! H 30 + + P O! 4.4 x 1 0 - 13
2
Hydrogen sulfide ion HS - + H 20 <=! H 30 + + s - l . 3 x 10 - 1 3
Water l H20 + H20 <=! H 30 + + o H 2 l . O x 1 0 - 14
Hydroxide ion OH - + H 20 <=? H 30 + + 0 - < l Q - 3 6
a The reactions are of the form HA + H20(1) <==! H30 + (aq) + A - ( aq). The equilibrium (or acid-dissociation or ioniza
tion) constant is defined as
- [H30 + (aq)] [ A - (aq)]
°'
l..;.J Kc = K .
[HA][H20(I)]
where H20(1) = I . The equilibrium constant is often expressed in an analogous way to that used for pH, namely ,
-
pK. = - logK •. The base-dissociation constant Kb, for the conjugate base, A ( aq), of HA is given by
Kb = . OO I x 10 - 14/K. at 25°C ; see Eq. (5 . 1 3).