Page 180 - Basic physical chemistry for the atmospheric sciences
P. 180
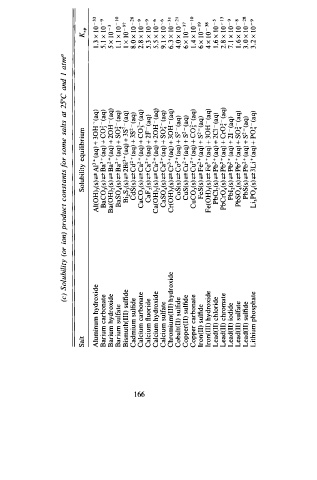
(c) Solubility (or ion) product constants f o r some salts at 25°C and I atm0
Salt Solubility equilibrium K sp
Aluminum hydroxide Al(0Hh(s) <=Z Al3 ( aq) + 30H - (aq) 1 . 3 x 1 0 - 3 3
+
Barium carbonate BaCOh) <2 Ba3 + (aq) + CO� - ( aq) 5 . I x l 0 - 9
Barium hydroxide Ba(OH)z(s) ;:± Ba2 + (aq) + 20H - ( aq) 5 x 1 0 - 3
+
Barium sulfate BaS04(s) <=Z B a2 ( aq) + SO� - ( aq) I . I x l 0 - 10
Bismuth(III) sulfide Bi2S (s) <=Z 2 Bi3 + (aq) + 3S2 - ( aq) I x 1 0 - 9 7
3
Cadmium sulfide CdS(s) <=Z C d2 + (aq) + 3s2 - ( aq) 8 . 0 x 1 0 - 2 8
......
°' Calcium carbonate CaC03(s) <=Z C a2 + (aq) + CO�- (aq) 2.S x 1 0 - 9
°'
Calcium fluoride CaFi(s) <=Z C a2 + (aq) + F 2 - (aq) 5 . 3 x 1 0 - 9
Calcium hydroxide Ca(OH)z(s) ;:± C a2 + (aq) + 20H - (aq) 5 . 5 x 1 0 - 6
Calcium sulfate CaS04(s) ;:± Ca2 + (aq) + SO� - (aq) 9. 1 x 1 0 - 6
Chromium(III) hydroxide Cr(OHh(s) <=Z Cr3 + (aq) + 30H - ( aq) 6 . 3 x 1 0 - 3 1
2
Cobalt(ll) sulfide CoS(s) <=Z C 0 + ( aq) + s2 - ( aq) 4. x 0 1 0 - 2 1
Copper(II) sulfide CuS(s) µ Cu2 + (aq) + S2 - (aq) 6 x 1 0 - 37
Copper carbonate CuC0 (s) <=Z C u2 + ( aq) + co�- (aq) I . 4 x 1 0 - 10
3
Iron(ll) sulfide FeS(s) � Fe2 + (aq) + s2 - (aq) 6 x 1 0 - 19
lron(III) hydroxide Fe(OHh(s) ;:± Fe3 + (aq) + 30H - ( aq) 4 x 1 0 - 38
Lead(ll) chloride PbCl2(s) � P b2 + (aq) + 2c1 - (aq) 1 . 6 X 1 0 - 5
Lead(ll) chromate PbCr04(s) � P b2 + (aq) + Cro� - (aq) 2 . S x 1 0 - 13
-
Lead(II) iodide Pbli(s) � Pb2 + (aq) + 21 ( aq) 7. 1 x 1 0 - 9
-
Lead(ll) sulfate PbSOh) <2 Pb2 + (aq) + SO� ( aq) J . 6 X l 0 - B
2
Lead(II) sulfide PbS(s) � P b2 + (aq) + s - ( aq) 3 . 0 x 1 0 - 2 8
Lithium phosphate Li3PO/s) � 3Li ( aq) + Po� - (aq) 3 . 2 x l0 - 9
+