Page 183 - Basic physical chemistry for the atmospheric sciences
P. 183
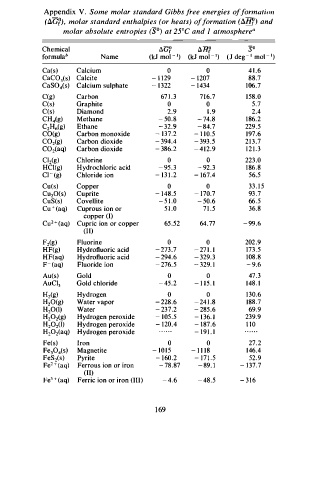
Appendix V . Some molar standard Gibbs f r ee energies o f f o rmation
(aG?). molar standard enthalpies (or heats) o f f o rmation (SHI() and
molar absolute entropies (S0) at 25°C and I atmosphere"
Chemical �G? �ll? so
formulab Name (kJ mo - l 1 ) (kJ mol - 1 ) ( J deg - • mo - l 1 )
Ca(s) Calcium 0 0 4 1 . 6
CaCOh) Calcite - 1 1 2 9 - 1 207 88.7
CaSOis) Calcium sulphate - 1 322 - 1 434 1 0 6.7
C(g) Carbon 67 1 . 3 7 1 6 .7 1 5 8.0
C(s) Graphite 0 0 5.7
C(s) Diamond 2 .9 1 . 9 2.4
CHig) Methane - 5 0 . 8 - 7 4.8 1 86.2
C2H6(g) Ethane - 3 2.9 - 8 4.7 229.5
CO(g) Carbon monoxide - 1 37.2 - l l 0.5 1 9 7.6
C02 (g) Carbon dioxide - 3 94 4 - 3 93 .5 2 1 3 . 7
.
C02(aq) Carbon dioxide - 3 86.2 - 4 1 2.9 1 2 1 . 3
Cl2(g) Chlorine 0 0 223 .0
HCl(g) H y drochloric acid - 9 5 . 3 - 92. 3 1 8 6.8
ci - ( g) Chloride ion - 1 3 1 .2 - 167.4 56.5
Cu(s) Copper 0 0 33 . 1 5
Cu 20(s) Cuprite - 1 48.5 - 1 70.7 93 .7
CuS(s) Covellite - 5 1 . 0 - 5 0.6 66. 5
+
Cu ( aq) Cuprous ion or 5 1 . 0 7 1 .5 36.8
copper (I)
Cu2 + (aq) Cupric ion or copper 65 .52 64.77 - 99 .6
(II)
Fz (g) Fluorine 0 0 202 .9
HF(g) Hydrofluoric acid - 273 .7 - 2 7 1 . 1 173. 5
H F (aq) Hydrofluoric acid - 294 .6 - 329. 3 1 0 8.8
F- (aq) Fluoride ion - 2 76 5 - 3 29. I - 9.6
.
Au(s) Gold 0 0 47. 3
AuCl3 Gold chloride - 45 .2 - 1 1 5 . I 1 4 8. I
Hz (g) Hydrogen 0 0 1 3 0.6
H20(g) Water vapor - 2 28.6 - 2 4 1 . 8 188.7
H10(I) Water - 2 37.2 - 2 85.6 69.9
Hydrogen peroxide
H20z(g) - 1 05 .5 - 1 36. 1 239 .9
H20z(I) Hydrogen peroxide - 1 20.4 - 1 87.6 1 1 0
H202(aq) Hydrogen peroxide - 1 9 1 . 1
Fe(s) Iron 0 0 27. 2
Fe30is) Magnetite - 1 0 1 5 - 1 1 1 8 146.4
FeS (s) Pyrite - 1 60 . 2 - 1 7 1 . 5 52 .9
2
Fe2 + ( aq) Ferrous ion or iron - 7 8.87 - 8 9. 1 - 1 3 7 .7
(II)
Fe3 + (aq) Ferric ion or i ron (III) - 4 .6 - 48.5 - 3 1 6
1 6 9