Page 186 - Basic physical chemistry for the atmospheric sciences
P. 186
V
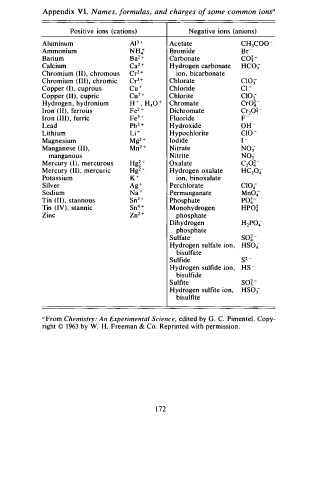
Appendix I . Names, f o rmulas, and charges o f some common ions"
Positive ions (cations) Negative ions (anions)
Aluminum AP + Acetate CH3Coo -
Ammonium NH4+ Bromide Br-
2
Barium Ba + Carbonate co� -
Calcium Ca + 2 Hydrogen carbonate Hco -
3
Chromium (II), chromous Cr2 + ion , bicarbonate
3
Chromium (III), chromic Cr + Chlorate CI03-
Copper (I), cuprous Cu + Chloride c1 -
Copper (II), cupric Cu2 + Chlorite CIO;
Hydrogen , hydronium H + , H30 + Chromate Cro� -
Iron (II), ferrous Fe2 + Di chromate Cr20� -
Iron (III) , ferric Fe3 + Fluoride p -
Lead Pb2 + Hydroxide OH -
Lithium Li + Hypochlorite c10 -
Magnesium Mg + Iodide 1 -
z
Manganese (II), Mn2 + Nitrate N03
manganous Nitrite NO;
Mercury (I), mercurous Hg � + Oxalate C26� -
Mercury (II), mercuric Hgz + Hydrogen oxalate HC204-
Potassium K + ion, binoxalate
Silver Ag + Perchlorate c104-
Sodium Na + Permanganate Mn04
Tin (II), stannous Sn2 + Phosphate PO� -
Tin (IV), stannic Sn4 + Monohydrogen HPO� -
Zinc Zn2 + phosphate
Dihydrogen H2P04-
phosphate
Sulfate so� -
Hydrogen sulfate ion , HS04-
bisulfate
Sulfide sz -
Hydrogen sulfide ion , Hs -
bisulfide
Sulfite soj -
H y drogen sulfite ion, HS03-
bisulfite
" F rom Chemistry: An Experimental Science, edited by G. C. Pimentel . Copy
right © 1 9 63 by W. H. Freeman & Co. Reprinted with permission .
1 7 2