Page 184 - Basic physical chemistry for the atmospheric sciences
P. 184
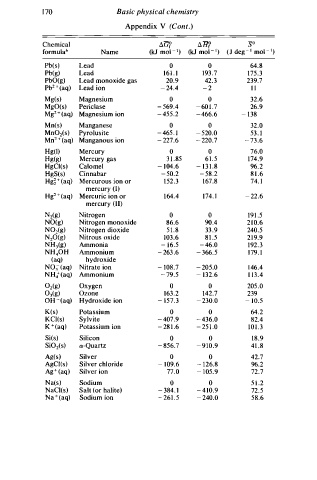
1 7 0 Basic physical chemistry
Appendix V (Cont.)
Chemical ilG? ilH? so
l
formulab Name (kJ mo - 1 ) (kJ mol - 1 ) ( J deg- 1 mol - 1 )
Pb(s) Lead 0 0 64.8
3
Pb(g) Lead 16 1 . 1 19 . 7 1 7 5 . 3
PbO(g) Lead monoxide gas 20.9 42. 3 239.7
2
Pb + ( aq) Lead ion - 2 4.4 - 2 1 1
Mg(s) Magnesium 0 0 32.6
MgO(s) Periclase - 5 69.4 - 6 0 1 . 7 26.9
Mg2 + (aq) Magnesium ion - 4 55.2 - 4 66.6 - 1 38
M n (s) Manganese 0 0 32.0
MnOz(s) Pyrolusite - 46 . 1 - 5 20.0 53 . l
5
Mn2 + (aq) Manganous ion - 2 27.6 - 2 20.7 - 73.6
Hg(l) Mercury 0 0 76.0
Hg(g) Mercury gas 3 1 . 85 6 1 . 5 1 7 4.9
HgCl(s) Calo me I - 1 04.6 - 1 3 1 . 8 96.2
HgS(s) Cinnabar - 5 0.2 - 58.2 8 1 . 6
Hg� + ( aq) Mercurous ion or 1 5 2 . 3 1 6 7.8 74. I
mercury (I)
Hg + (aq) Mercuric ion or 1 6 4.4 1 7 4. 1 - 2 2.6
2
mercury (II)
N z(g) Nitrogen 0 0 1 9 1 . 5
NO(g) Nitrogen monoxide 86.6 90.4 2 1 0 .6
N02(g) Nitrogen dioxide 5 1 . 8 33 .9 240.5
N20(g) Nitrous oxide 103.6 8 1 . 5 2 1 9.9
NH3(g) Ammonia - 1 6.5 - 4 6.0 192.3
NH40H Ammonium - 2 63 .6 - 3 66 5 1 7 9. l
.
(aq) hydroxide
N03 - (aq) Nitrate ion - 1 08.7 - 2 05.0 1 4 6.4
NH,;- (aq) Ammonium - 7 9.5 - 132.6 1 1 3 . 4
0 (g) Oxygen 0 0 205 .0
2
03(g) Ozone 16 . 2 142.7 239
3
OH - (aq) Hydroxide ion - 1 57.3 - 2 30.0 - 1 0.5
u
K(s) Potassi m 0 0 64.2
KCl(s) Sylvite - 4 07.9 - 4 36.0 82.4
K + (aq) Potassium ion - 2 8 1 . 6 - 2 5 1 .0 1 0 1 . 3
Si(s) Silicon 0 0 1 8 . 9
SiOz(s) a-Quartz - 8 56.7 - 9 10.9 41 .8
Ag(s) Silver 0 0 42.7
AgCl(s) Silver chloride - 1 09.6 - 1 26.8 %.2
Ag+ ( aq) Silver ion 77.0 - 1 05.9 72.7
Na(s) Sodium 0 0 5 1 .2
NaCl(s) Salt (or halite) - 3 84. l - 4 10.9 72.5
Na + (aq) Sodium ion - 2 61 . 5 - 2 40.0 58.6