Page 178 - Basic physical chemistry for the atmospheric sciences
P. 178
°'
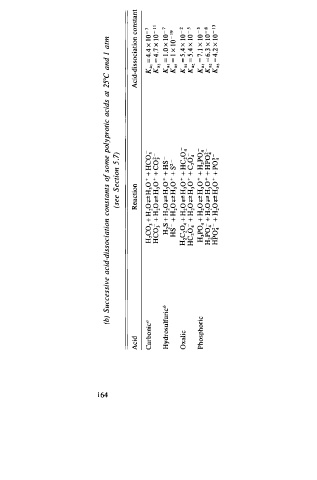
"""' (b) Successive acid-dissociation constants o f some polyprotic acids at 25°C and I atm
(see Section 5 . 7 )
Acid Reaction Acid-dissociation constant
Carbonic0 H2C03 + H20 <=Z H 30 + + HC03 K a1 = 4 .4 x 1 0 - 7 1 1
HC03 + H 20 <=Z H 30 + + coj - K a2 = 4 .7 X 1 0 -
Hydrosulfuricb H 2 S + H 2 0 <=Z H 30 + + H s Ka, = 1 .0 x 1 0 - 7
HS - + H 20 <=Z H 30 + + s 2 - Ka, = l x l 0 - 19
Oxalic H 2C 2 04 + H2 0 <=Z H 30 + + HC204 K a1 = 5 .4 x 1 0 - 2
HC204 + H z0 <=Z H 30+ + C204- K a2 = 5 .4 x 1 0 - 5
Phosphoric H3P04 + Hz0 <=Z H 30+ + H2P04 K a , = 7 . 1 x 1 0 - 3
1
H2P04 + H20 <=Z H 3 0 + + HPO� K a2 = 6. 3 X 0- s
HPO� - + H20 .,z H 30 + + P o� - Ka3 = 4 .2 x 1 0 - 1 3