Page 176 - Basic physical chemistry for the atmospheric sciences
P. 176
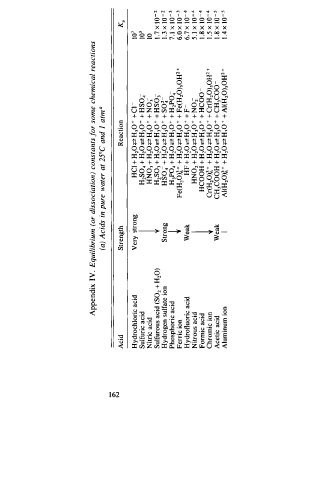
Appendix IV. Equilibrium (or dissociation) constants f o r some chemical reactions
m
(a) Acids in pure water at 25°C and I at a
-
O'I
N
Acid Strength Reaction K.
Hydrochloric acid Ve'}' rng HC + l H 2 0 � H30 + + c 1 - 1 0 1
Sulfuric acid H2S04 + H2 0 � H 3 0 + + HS04- 1 0 3
Nitric acid HN03 + H2 0 � H30 + + N 03- 1 0
Sulfurous acid (S02 + H20) H S03 + H 20 � H 30 + + H S03 l . 7 x 1 0 - 2
2
Hydrogen sulfate ion Strong HS04 + Hi O � H30 + + s o� 1 . 3 x 1 0 - 2
Phosphoric acid i H 3 P04 + H 20 � H 30 + + H 2 P04- 7. l x 1 0 - 3
2
Ferric ion Fe(H20)� + + H2 0 � H 30 + + Fe(H 0)50H + 6. x 1 0 - 3 4
0
2
Hydrofluoric acid Weak HF + H2 0 � H 30 + + F - 6.7 x 1 0 -
Nitrous acid HN0 2 + H 0 2 � H p + + NOi 5. 1 x 1 0 - 4
Formic acid l HCOOH + H2 0 � H 3 0 + + HCoo 1 . 8 x 1 0 - 4
2
Chromic ion Cr(H 0)� + + H 20 � H p + + C r(H 0)50H + 1 . 5 x 1 0 - 4
2
2
Acetic acid Weak CH3COOH + H 2 0 � H30 + + C H3Coo l . 8 x l0 - 5
2
Aluminum ion I Al(H20)� + + H1 0 � H,o + + Al(H20)sOH + l . 4 x 1 0 - 5