Page 133 - Battery Reference Book
P. 133
Nickel-metal hydride secondary batteries 4/17
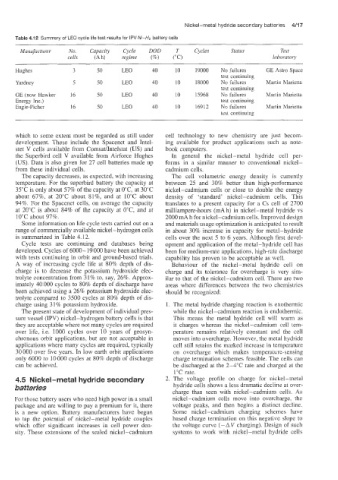
Table 4.12 Summary of LEO cycle life test results for IPV Ni-H2 battery cells
Manufacturer No. Capacity Cycle DOD T Cycles Status Test
cells (Ah) reginze (%) ("C) laborator):
Hughes 3 50 LEO 40 10 19000 No failures GE Astro Space
test continuing
Yardney 5 50 LEO 40 10 18000 No failures Martin Marietta
test continuing
GE (now Hawker 16 50 LEO 40 10 15968 No failures Martin Marietta
Energy Inc.) test continuing
Eagle-Picher 16 50 LEO 40 10 16912 No failures Martin Marietta
test continuing
which to some extent must be regarded as still under cell technology to new chemistry are just becom-
development. 'These include the Spacenet and Intel- ing available for product applications such as note-
stet V cells available from Comsaflntelstat (US) and book computers.
the Superbird cell V available from Airforce Hughes In general the nickel-metal hydnde cell per-
(US). Data is also given for 27 cell batteries made up forms in a similar manner to conventional nickel-
from these individual cells. cadmium cells.
The capacity decreases, as expected, with increasing The cell volumetric energy density is currently
temperature. For the superbird battery the capacity at between 25 and 30% better than high-performance
35°C is only about 57% of the capacity at WC, at 30°C nickel-cadmium cells or close to double the energy
about 6796, at 20°C about 81%, and at 10°C about density of 'standard' nickel-cadmium cells. This
94%. For the Spacenet cells, on average the capacity translates to a present capacity for a Cs cell of 2700
at 20°C is about 84% of the capacity at WC, and at milliampere-hours (mA h) in nickel-metal hydride vs
lWC about 97'%/0. 2000 mA h for nickel-cadmium cells. Improved design
Some information on life cycle tests carried out on a and materials usage optimization is anticipated to result
range of commercially available nickel-hydrogen cells in about 30% increase in capacity for metal-hydride
is summarized in Table 4.12. cells over the next 5 to 6 years. Although first devel-
Cycle tests are continuing and databases being opment and application of the metal-hydride cell has
developed. Cycles of 6000- 19 0100 have been achieved been for medium-rate applications, high-rate discharge
with tests continuing in orbit and ground-based trials. capability has proven to be acceptable as well.
A way of increasing cycle life at 80% depth of dis- Behaviour of the nickel-metal hydride cell on
charge is to decrease the potassium hydroxide elec- charge and its toIerance for overcharge is very sim-
trolyte concentration from 3 1% to, say, 26%. Approx- ilar to that of the nickel-cadmium cell. There are two
imately 40 000 cycles to 80% depth of discharge have areas where differences between the two chemistries
been achieved using a 26% potassium hydroxide elec- should be recognized:
trolyte compared to 3500 cycles at 80% depth of dis-
charge using 3 1 % potassium hydroxide. 1. The metal hydride charging reaction is exothermic
The present state of development of individual pres- while the nickel-cadmium reaction is endothermic.
sure vessel (PV) nickel-hydrogen battery cells is that This means the metal hydride cell will warm as
they are acceptable where not many cycles are required it charges whereas the nickel-cadmium cell tem-
over life, Le. 1000 cycles over 10 years of geosyn- perature remains relatively constant and the cell
chronous orbit applications, but are not acceptable in moves into overcharge. However, the metal hydride
applications wlhere many cycles are required, typically cell still retains the marked increase in temperature
30 000 over five years. In low earth orbit applications on overcharge which makes temperature-sensing
only 6000 to 10000 cycles at 80% depth of discharge charge termination schemes feasible. The cells can
can be achieved. be discharged at the 2-4T rate and charged at the
1°C rate.
4.5 Nickel-metal hydride secondary 2. The voltage profile on charge for nickel-metal
atteries hydride cells shows a less dramatic decline at over-
charge than seen with nickel-cadmium cells. As
For those battery users who need high power in a small nickel-cadmium cells move into overcharge, the
package and are willing to pay a premium for it, there voltage peaks, and then begins a distinct decline.
is a new opti'on. Battery manufacturers have begun Some nickel-cadmium charging schemes have
to tap the potential of nickel-metal hydride couples based charge termination on this negative slope to
which offer significant increases in cell power den- the voltage curve (-AV charging). Design of such
sity. These extensions of the sealed nickel-cadmium systems to work with nickel-metal hydride cells