Page 129 - Battery Reference Book
P. 129
Nickel-iron secondary batteries 4/13
0 3 6 9 12 15 18 21 24
Standing time (months)
Figure 4.10 Self-discharge of Dryfit batteries at different temper-
atures compared with standard battery in lead-antimony alloy.
1, Dryfit battery (calcium-lead alloy); 2, conventional antimonial
0 2 4 6 8 1 0 1 2 lead-acid battery (Courtesy of Dryfit)
Capacity (Ah)
Figure 4.8 Maximum allowable permanent discharge currents:
Dryfit and nickel-c.admium batteries with mass electrodes and
sintered electrodes: 1, Dryfit; 2, nickel-cadmium with sintered
electrodes; 3, niclqei-cadmium with mass electrodes (varta)
(Courtesy of Dryfit)
25
W a
> 100 -20 "C I I I I I I ' l l l l l
.- 0 2 4 6 8 10 12 14 16 18 20 22 24
c
0
g 75 - Standing time (months)
m
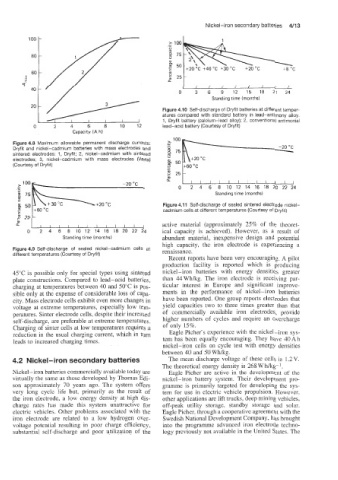
0°C ------m +20 OC Figure 4.1 1 Self-discharge of sealed sintered electrode nickel-
cadmium cells at different temperatures (Courtesy of Dryfit)
W 251
active material (approximately 25% of the theoret-
a I l l l l l l l l l ~ l l
0 2 4 6 8 10 12 14 16 18 20 22 24 ical capacity is achieved). However, as a result of
Standing time (months) abundant material, inexpensive design and potential
high capacity, the iron electrode is experiencing a
Figure 4.9 Self-discharge of sealed nickel-cadmium cells at renaissance.
different temperatures (Courtesy of Dryfit)
Recent reports have been very encouraging. A pilot
production facility is reported which is producing
45°C is possible only for special types using sintered nickel-iron batteries with energy densities greater
plate constructions. Compared to lead-acid batteries, than 44Whkg. The iron electrode is receiving par-
charging at temperatures between 40 and 50°C is pos- ticular interest in Europe and significant improve-
sible only at the expense of considerable loss of capa- ments in the performance of nickel-iron batteries
city. Mass elect]-ode cells exhibit even more changes in have been reported. One group reports electrodes that
voltage at extreme temperatures, especially low tem- yield capacities two to three times greater than that
peratures. Sinter electrode cells, despite their increased of commercially available iron electrodes, provide
self-discharge, are preferable at extreme temperatures. higher numbers of cycles and require an overcharge
Charging of sinier cells at low temperatures requires a of only 15%.
reduction in the usual charging current, which in turn Eagle Picher's experience with the nickel-iron sys-
leads to increased charging times. tem has been equally encouraging. They have 40 Ah
nickel-iron cells on cycle test with energy densities
between 40 and 50 Whikg.
ickel-iron secondary batteries The mean discharge voltage of these cells is 1.2V.
The theoretical energy density is 268 W h/kg-'.
Nickel-iron batteries commercially available today are Eagle Picher are active in the development of the
virtually the same as those developed by Thomas Edi- nickel-iron battery system. Their development pro-
son approximately 70 years ago. The system offers gramme is primarily targeted for developing the sys-
very long cyclie life but, primarily as the result of tem for use in electric vehicle propulsion. However,
the iron electrode, a low energy density at high dis- other applications are lift trucks, deep mining vehicles,
charge rates has made this system unattractive for off-peak utility storage, standby storage and solar.
electric vehicles. Other problems associated with the Eagle Picher, through a cooperative agreement with the
iron electrode are related to a low hydrogen over- Swedish National Development Company, has brought
voltage potential resulting in poor charge efficiency, into the programme advanced iron electrode techno-
substantial self-discharge and poor utilization of the logy previously not available in the United States. The