Page 174 - Battery Reference Book
P. 174
9/14 Lithium batteries
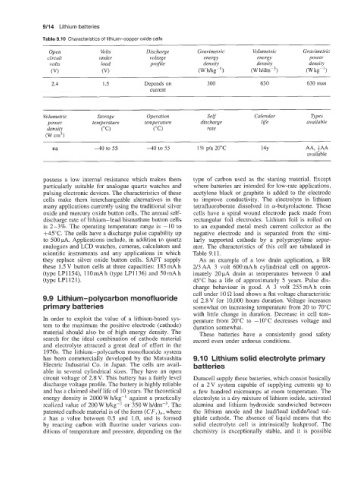
Table 9.10 Characteristics of lithium-copper oxide cells
Open Volts Discharge Gravimetric Volumetric Gravimetric
circuit under voltage energy energy power
volts load projile density density density
(VI (VI (Wmg-') (Wh/dmp3) (Wkg-')
2.4 1.5 Depends on 300 630 630 max
current
Volumetric Storage Operation Self Calendar Types
power temperature temperature discharge life available
density ("C) (" C) rate
(W cm3)
na -40 to 55 -40 to 55 1% p/a 20°C 14Y AA, iAA
available
possess a low internal resistance which makes them type of carbon used as the starting material. Except
particularly suitable for analogue quartz watches and where batteries are intended for low-rate applications,
pulsing electronic devices. The characteristics of these acetylene black or graphite is added to the electrode
cells make them interchangeable alternatives in the to improve conductivity. The electrolyte is lithium
many applications currently using the traditional silver tetrafluoroborate dissolved in a-butyrolactone. These
oxide and mercury oxide button cells. The annual self- cells have a spiral wound electrode pack made from
discharge rate of lithium-lead bismuthate button cells rectangular foil electrodes. Lithium foil is rolled on
is 2-3%. The operating temperature range is -10 to to an expanded metal mesh current collector as the
f45"C. The cells have a discharge pulse capability up negative electrode and is separated from the simi-
to 500 PA. Applications include, in addition to quartz larly supported cathode by a polypropylene separ-
analogues and LCD watches, cameras, calculators and ator. The characteristics of this cell are tabulated in
scientific instruments and any applications in which Table 9.11.
they replace silver oxide button cells. SAFT supply As an example of a low drain application, a BR
these 1.5V button cells at three capacities: 185mAh 2/3AA 3 volt 600mAh cylindrical cell on approx-
(type LP1154), llOmAh (type LP1136) and 5OmAh imately 20 yA drain at temperatures between 0 and
(type LP1121). 45°C has a life of approximately 5 years. Pulse dis-
charge behaviour is good. A 3 volt 255mAh coin
cell under 10 C2 load shows a flat voltage characteristic
9.9 Lithium-polycarbon monofluoride of 2.8V for 10,000 hours duration. Voltage increases
primary batteries somewhat on increasing temperature from 20 to 70°C
with little change in duration. Decrease in cell tem-
In order to exploit the value of a lithium-based sys- perature from 20°C to -10°C decreases voltage and
tem to the maximum the positive electrode (cathode) duration somewhat.
material should also be of high energy density. The These batteries have a consistently good safety
search for the ideal combination of cathode material record even under arduous conditions.
and electrolyte attracted a great deal of effort in the
1970s. The lithium-polycarbon monofluoride system
has been commercially developed by the Matsushita 9.1 0 Lithium solid electrolyte primary
Electric Industrial Co. in Japan. The cells are avail- batteries
able in several cylindrical sizes. They have an open
circuit voltage of 2.8 V. This battery has a fairly level Duracell supply these batteries, which consist basically
discharge voltage profile. The battery is highly reliable of a 2V system capable of supplying currents up to
and has a claimed shelf life of 10 years. The theoretical a few hundred microamps at room temperature. The
energy density is 2000 W h/kg-' against a practically electrolyte is a dry mixture of lithium iodide, activated
realized value of 200 W h/kg-' or 350 W h/dm-3. The alumina and lithium hydroxide sandwiched between
patented cathode material is of the form (CF,), , where the lithium anode and the lead/lead iodide/lead sul-
x has a value between 0.5 and 1.0, and is formed phide cathode. The absence of liquid means that the
by reacting carbon with fluorine under various con- solid electrolyte cell is intrinsically leakproof. The
ditions of temperature and pressure, depending on the chemistry is exceptionally stable, and it is possible