Page 176 - Battery Reference Book
P. 176
9/16 Lithium batteries
voltage reversal. No explosion due to pressure build- 5. The battery is a relatively high-impedance device,
up or chemical reaction can occur. Prolonged short- so short-circuit currents are of relatively low mag-
circuiting will in fact result in a separation between nitude. However, discharge of this system depends
the electrode and the electrolyte, rendering the cell on slow solid-state diffusion phenomena. Too rapid
inoperative. discharge, such as shunting, involves such rapid
The following handling procedures and precautions lithium solution at the anode to electrolyte inter-
are recommended: face that vacancies may be produced irreversibly.
Extended shorting, for example periods of the order
The solid electrolyte battery can be discharged at of 30min, can render a cell completely inoperable.
temperatures up to 125°C and stored, with little loss 6. Care must be exercised in handling, lead attach-
in capacity, at temperatures up to 200°C. However, ment, etc., to prevent mechanical damage to her-
for best performance, excessively high temperatures metic glass seal feedthroughs. Loss of hermeticity
should be avoided. Temperatures above 200°C may will ultimately make the battery completely in-
cause bulging and failure of the hermetic seal. The operable.
cell should not be incinerated unless suitable pro- 7. Cells should not be opened, crushed, punctured or
cedures are followed and appropriate precautions otherwise mutilated.
taken at the disposal site.
High-impedance voltmeters must be used for The performance characteristics of lithium solid elec-
measurement, preferably with lo9 Q or higher input trolyte batteries are shown in Table 9.13.
impedances. Batteries with open circuit voltages
of 1OV or less may be checked rapidly using The Hanvell development lithium V6O13 solid state
meters with lo7 Q input impedance. Prolonged battery
measurements using such instruments will result in This cell can be discharged from 2.7 to 1.7V at the
gradual voltage decrease with time due to battery C/10 rate (0.2mA~m-~) giving 100% of theoretical
polarization. cell capacity. More than 50% of theoretical cell
Care must be exercised to prevent shunting of bat- capacity is available at the C rate, 80% at the C/2
tery terminals by the human body (fingers, hands, rate and 100% at the C/4 rate. Electrodes of area
etc.). Such shunting can correspond to a load res- 40cm2 give 80mAh capacity. The energy density of
istance of the order of lo5 Q, which will load down the cell is 21 1 W h/kg-'. These cells can be operated
the battery accordingly. The higher the battery volt- at temperatures up to 120°C.
age, the greater the transient effect of such hand-
ling and the longer the time subsequently required The Mallory discontinued lithium-titanium disulphide
for recovery to full open-circuit voltage level. No secondary battery
permanent damage occurs in such accidental short-
duration shunting. Despite its high practical energy density (280
Batteries must be protected against ambient high- Wag-'), high voltage (2.5V) and good cycling
humidity environments, which might pass through behaviour, due mainly to lack of durability,
dew point resulting in moisture condensation across commercial production of this solid state may
the battery terminals. Such condensation would never be commenced. A similar type of secondary
electrically shunt, shorting out the battery. battery produced by Eveready featuring an inorganic
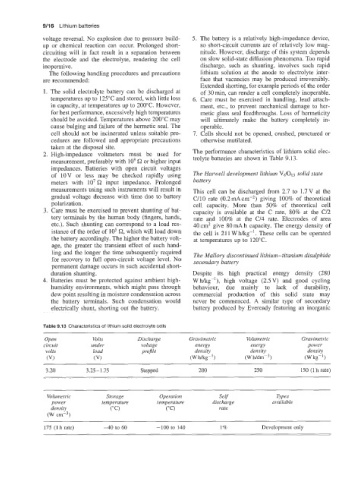
Table 9.13 Characteristics of lithium solid electrolyte cells
Open Volts Discharge Gravimetric Volumetric Gravimetric
circuit under voltage energy energy power
volts load projile density density density
(VI (VI (W fig-') (Wh/dmb3) (W kg- 1
3.20 3.25- 1.75 Stepped 200 250 150 (1 h rate)
Volumetric Storage Operation Self Types
power temperature temperature discharge available
density ("C) ("C) rate
(W crnp3)
175 (1 h rate) -40 to 60 -100 to 140 1% Development only