Page 43 - Battery Reference Book
P. 43
1/28 Introduction to battery technology
0.5
Sulphuric acid concentration (% w/w)
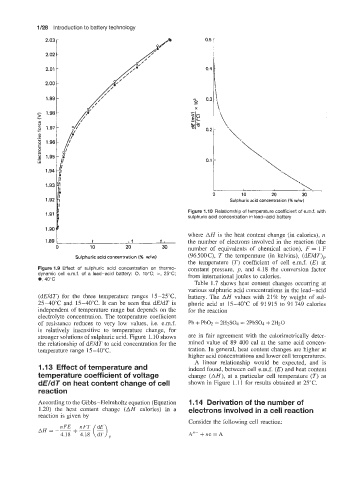
Figure 1.10 Relationship of temperature coefficient of e.m.f. with
sulphuric acid concentration in lead-acid battery
1.89 1 t It t1 where AH is the heat content change (in calories), n
the number of electrons involved in the reaction (the
0 10 20 30 number of equivalents of chemical action), F = 1 F
Sulphuric acid concentration (% w/w) (96 500C), T the temperature (in kelvins), (dEldT),
the temperature (T) coefficient of cell e.m.f. (E) at
Figure 1.9 Effect of sulphuric acid concentration on thermo- constant pressure, p, and 4.18 the conversion factor
dynamic cell e.rn.f. of a lead-acid battery: 0, 15°C; x, 25°C; from international joules to calories.
e, 40°C
Table 1.7 shows heat content changes occurring at
various sulphuric acid concentrations in the lead-acid
(dEldT) for the three temperature ranges 15-25"C, battery. The AH values with 21% by weight of sul-
2540°C and 15-40°C. It can be seen that dEldT is phuric acid at 15-40°C of 91 915 to 91 749 calories
independent of temperature range but depends on the for the reaction
electrolyte concentration. The temperature coefficient
of resistance reduces to very low values, i.e. e.m.f. Pb + PbO2 + 2H2SO4 = 2PbSO4 + 2H20
is relatively insensitive to temperature change, for
stronger solutions of sulphuric acid. Figure 1.10 shows are in fair agreement with the calorimetrically deter-
the relationship of dEldT to acid concentration for the mined value of 89 400 cal at the same acid concen-
temperature range 15-40°C. tration. In general, heat content changes are higher at
higher acid concentrations and lower cell temperatures.
A linear relationship would be expected, and is
1.13 Effect of temperature and indeed found, between cell e.m.f. (E) and heat content
temperature coefficient of voltage change (AH), at a particular cell temperature (T) as
d€/dT on heat content change of cell shown in Figure 1.11 for results obtained at 25°C.
reaction
4
According to the Gibbs-Helmholtz equation (Equation 1 .I Derivation of the number of
1.20) the heat content change (AH calories) in a electrons involved in a cell reaction
reaction is given by Consider the following cell reaction:
nFE
AH = ___ f (!?!!I
4.18 4.18 dT , An' + ne = A