Page 45 - Battery Reference Book
P. 45
1/30 Introduction to battery technology
of 100% sulphuric acid is 1.8305) and 2 mol of water
are produced increasing the volume of electrolyte by 25 261
36ml. Thus the net volume reduction in the elec-
trolyte accompanying the production of 93 000 C is S L
107.07 - 36 = 71.07 ml. This electrolyte volume is, of P 24
course, recovered during the next charge cycle when g
the sulphuric acid is regenerated.
By definition, the quoted values of AH and .! 8 231
193 000 C (2 F) refer to the above reaction in which 22
1 mol of lead and of lead dioxide react with 2mol of
sulphuric acid. p- 211
Knowing AH, E, dEldT and the cell temperature 0
0
(in kelvins) it is possible to calculate the ampere hour .-
L
P -
capacity (C) of this cell from Equation 1.18: 2 20-
a
0 19 -
P
8
2 18-
3600 x C 0
- U
-
4.18 17-
i.e. nF = 3600C. Therefore -
16
c=- 4.18 x AH (1.76) 15
3600 [. - T (g )] 0 1000 2000 3000 4000 5000
Initial volume of sulphuric acid (ml)
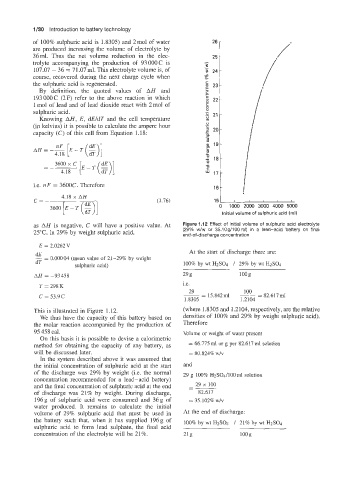
as AH is negative, C will have a positive value. At Figure 1.12 Effect of initial volume of sulphuric acid electrolyte
2YC, in 29% by weight sulphuric acid, (29% w/w or 35.10g/100ml) in a lead-acid battery on final
end-of-discharge concentration
E = 2.0262 V
dE At the start of discharge there are:
- = 0.00004 (mean value of 21-29% by weight
dT 100% by wt H2S04 / 29% by wt HzSO4
sulphuric acid)
AH = -93458 29 g 100 g
T = 298K i.e.
c = 53.9 c -- - 15.842ml __ -82.617ml
29
-
loo
1.8305 1.2104
This is illustrated in Figure 1.12. (where 1.8305 and 1.2104, respectively, are the relative
We thus have the capacity of this battery based on densities of 100% and 29% by weight sulphuric acid).
the molar reaction accompanied by the production of Therefore
95 458 cal. Volume or weight of water present
On this basis it is possible to devise a calorimetric
method for obtaining the capacity of any battery, as = 66.775 ml or g per 82.617 ml solution
will be discussed later. = 80.824% W/V
In the system described above it was assumed that
the initial concentration of sulphuric acid at the start and
of the discharge was 29% by weight (i.e. the normal 29 g 100% HzS04/100ml solution
concentration recommended for a lead-acid battery)
29 x 100
and the final concentration of sulphuric acid at the end --
-
of discharge was 21% by weight. During discharge, 82.617
196g of sulphuric acid were consumed and 36g of = 35.102% W/V
water produced. It remains to calculate the initial
volume of 29% sulphuric acid that must be used in At the end of discharge:
the battery such that, when it has supplied 196g of 100% by wt HzS04 / 21% by wt HzSO4
sulphuric acid to form lead sulphate, the final acid
concentration of the electrolyte will be 21%. 21 g 100 g