Page 42 - Battery Reference Book
P. 42
Effect of cell temperature on e.m.f. in the lead-acid battery 1/27
charge /
2.4 . Practical
thermodynamic
charge Discharge
i
1.4 L I I I I I I I I
0 10 20 30 40 50 60 70 80 90
Time (min)
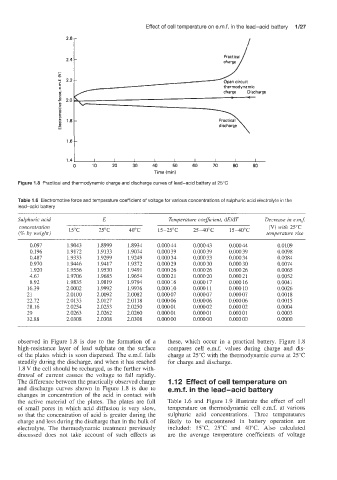
Figure 1.8 Practical and thermodynamic charge and discharge curves of lead-acid battery at 25°C
Table 1.6 Electromotive force and temperature coefficient of voltage for various concentrations of sulphuric acid electrolfle in the
lead-acid battery
Sulphuric acid E Temperature coeficient, dEldT Decrease in e.m$
concentration 15°C (V) with 25°C
(5% by weight) 25°C 40°C 15-25°C 25-40°C 15-40°C temperature rise
0.097 1.9043 1.8999 1.8934 0.000 44 0.000 43 0.000 44 0.0109
0.196 1.9172 1.9133 1.9074 0.000 39 0.000 39 0.000 39 0.0098
0.487 1.9333 1.9299 1.9249 0.000 34 0.000 33 0.000 34 0.0084
0.970 1.9446 1.9417 1.9372 0.000 29 0.000 30 0.000 30 0.0074
1.920 1.9556 1.9530 1.9491 0.000 26 0.000 26 0.000 26 0.0065
4.67 1.9706 1.9685 1.9654 0.000 21 0.000 20 0.000 21 0.0852
8.92 1.9835 1.9819 1.9794 0.000 16 0.000 17 0.000 16 0.0041
16.39 2.0002 1.9992 1.9976 0.000 10 0.000 11 0.000 10 0.0026
21 2.0100 2.0092 2.0082 0.000 07 0.000 07 0.000 07 0.0018
22.72 2.0133 2.0127 2.0118 0.000 06 0.000 06 0.000 06 0.0015
28.16 2.0254 2.0253 2.0250 0.00001 0.000 02 0.000 02 0.0004
29 2.0263 2.0262 2.0260 0.000 01 0.000 01 0.000 01 0.0003
32.88 2.0308 2.0308 2.0308 0.000 00 0.000 00 0.00000 0.0000
observed in Figure 1.8 is due to the formation of a these, which occur in a practical battery. Figure 1.8
high-resistance layer of lead sulphate on the surface compares cell e.m.f. values during charge and dis-
of the plates which is soon dispersed. The e.m.f. falls charge at 25°C with the thermodynamic curve at 25°C
steadily during the discharge, and when it has reached for charge and discharge.
1.8 V the cell should be recharged, as the further with-
drawal of current causes the voltage to fall rapidly.
The difference between the practically observed charge 1.1 2 Effect of cell temperature o
and discharge curves shown in Figure 1.8 is due to e.m.f. in the lead-acid battery
changes in concentration of the acid in contact with
the active material of the plates. The plates are full Table 1.6 and Figure 1.9 illustrate the effect of cell
of small pores in which acid diffusion is very slow, temperature on thermodynamic cell e.m.f. at various
so that the concentration of acid is greater during the sulphuric acid concentrations. Three temperatures
charge and less during the discharge than in the bulk of likely to be encountered in battery operation are
electrolyte. The thermodynamic treatment previously included: WC, 25°C and 40°C. Also calculated
discussed does not take acco'unt of such effects as are the average temperature coefficients of voltage