Page 470 - Battery Reference Book
P. 470
Charge control and charge monitoring of seaied nickel-cadmium batteries 47/3
When recharge time is critical and it is desirable to 2.8
determine the amount of capacity restored to the bat- 250 mA
tery, recharge with constant current is recommended. 2.7 A
Constant-current charging is suitable when the dis-
charged ampere hours taken out in the previous dis-
charge cycle are known. It is necessary to monitor 2.6
battery voltage or manually cut off the current at the
end of charge to ensure a good service life for the
battery. This type of charging requires regulation by
means of an automatic timer. A normal recharge time
of 20 h is recommended, whereby the constant-current
charging rate is established to give an input of 110%
of the previous output. If necessary, the charge time
can be decreased, although it will require an increase
in charging current such that
Amperes x Holurs = 1 .I x Discharge output (Ah)
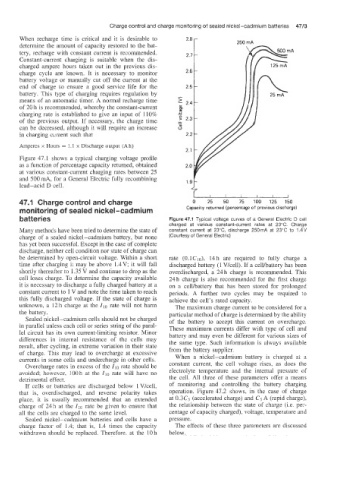
Figure 47.1 shows a typical charging voltage profile
as a function of percentage capacity returned, obtained
at various constant-current charging rates between 25
and 500 mA, for a General Electric fully recombining
lead-acid D cell.
7.1 Charge control and charge 0 25 50 75 100 125 150
monitoring of sealed nickel-cadmium Capacity returned (percentage of previous discharge)
batteries Figure 47.1 Typical voltage curves of a General Electric D cell
charged at various constant-current rates at 23°C. Charge
Many methods have been tried to determine the state of constant current at 23”C, discharge 250mA at 23’C to 1.4V
charge of a sealed nickel-cadmium battery, but none (Courtesy of General Electric)
has yet been successful. Except in the case of complete
discharge, nei.ther cell condition nor state of charge can
be determined by open-circuit voltage. Within a short rate (Q.lClo), 14h are required to fully charge a
time after charging it may be above 1.4V; it will fall discharged battery (1 Vkell). If a celllbattery has been
shortly thereafter to 1.35 V and continue to drop as the overdischarged, a 24 h charge is recommended. This
cell loses charge. To determine the capacity available 24 h charge is also recommended for the first charge
it is necessary to discharge a fully charged battery at a on a cellhattery that has been stored for prolonged
constant current to 1 V and note the time taken to reach periods. A further two cycles may be required to
this fully discharged voltage. If the state of charge is achieve the cell’s rated capacity.
unknown, a 12 h charge at the Zl0 rate will not harm The maximum charge current to be considered for a
the battery. particular method of charge is determined by the ability
Sealed nickel-cadmium cells should not be charged of the battery to accept this current on overcharge.
in parallel unless each cell or series string of the paral- These maximum currents differ with type of cell and
lel circuit has its own current-limiting resistor. Minor battery and may even be different for various sizes of
differences in internal resistance of the cells may the same type. Such information is always available
result, after cycling, in extreme variation in their state
of charge. This may lead to overcharge at excessive from the battery supplier.
currents in some cells and undercharge in other cells. When a nickel-cadmium battery is charged at a
Overcharge rates in excess of the I10 rate should be constant current, the cell voltage rises, as does the
avoided; however, 100h at the Ilo rate will have no electrolyte temperature and the internal pressure of
detrimental effect. the cell. All three of these parameters offer a means
If cells or batteries are discharged below lV/cell, of monitoring and controlling the battery charging
that is, overdischarged, and reverse polarity takes operation. Figure 47.2 shows, in the case of charge
place, it is usually recommended that an extended at 0.3Gj (accelerated charge) and Cs A (rapid charge),
charge of 24 h at the Ilo rate be given to ensure that the relationship between the state of charge (Le. per-
all the cells are charged to the same level. centage of capacity charged), voltage, temperature and
Sealed nickel-cadmium batteries and cells have a pressure.
charge factor of 1.4; that is, 1.4 times the capacity The effects of these three parameters are discussed
withdrawn should be replaced. Therefore, at the 10 h below.