Page 143 - Bio Engineering Approaches to Cancer Diagnosis and Treatment
P. 143
6.3 Photodynamic therapy 141
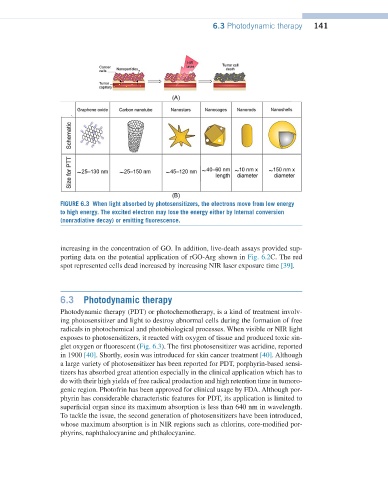
FIGURE 6.3 When light absorbed by photosensitizers, the electrons move from low energy
to high energy. The excited electron may lose the energy either by Internal conversion
(nonradiative decay) or emitting fluorescence.
increasing in the concentration of GO. In addition, live-death assays provided sup-
porting data on the potential application of rGO-Arg shown in Fig. 6.2C. The red
spot represented cells dead increased by increasing NIR laser exposure time [39].
6.3 Photodynamic therapy
Photodynamic therapy (PDT) or photochemotherapy, is a kind of treatment involv-
ing photosensitizer and light to destroy abnormal cells during the formation of free
radicals in photochemical and photobiological processes. When visible or NIR light
exposes to photosensitizers, it reacted with oxygen of tissue and produced toxic sin-
glet oxygen or fluorescent (Fig. 6.3). The first photosensitizer was acridine, reported
in 1900 [40]. Shortly, eosin was introduced for skin cancer treatment [40]. Although
a large variety of photosensitizer has been reported for PDT, porphyrin-based sensi-
tizers has absorbed great attention especially in the clinical application which has to
do with their high yields of free radical production and high retention time in tumoro-
genic region. Photofrin has been approved for clinical usage by FDA. Although por-
phyrin has considerable characteristic features for PDT, its application is limited to
superficial organ since its maximum absorption is less than 640 nm in wavelength.
To tackle the issue, the second generation of photosensitizers have been introduced,
whose maximum absorption is in NIR regions such as chlorins, core-modified por-
phyrins, naphthalocyanine and phthalocyanine.