Page 141 - Cascade_Biocatalysis_Integrating_Stereoselective_and_Environmentally_Friendly_Reactions
P. 141
5.3 Artificial Cascade Reactions Involving P450s 117
The direct oxidation of cycloalkanes into corresponding cyclic ketones with a
P450 and an ADH has been reported [105]. Cycloalkanes were initially hydroxylated
to the corresponding cycloalcohols by P450 BM3 mutants and then oxidized further
by an ADH to the ketones while simultaneous regeneration of NADPH consumed
by P450 BM3 was performed. The authors applied two different P450 BM3
mutants in combination with Lactobacillus kefir ADH [106] to establish conversion
of cyclohexane, cyclooctane and cyclodecane into the corresponding cyclic ketones.
The best results were obtained with P450 BM3 mutant 19A12 [107] and L.
kefir ADH, yielding product titers of 0.8 g l −1 (6.3 mM) cyclooctanone, 0.41 g l −1
cyclohexanone, and 0.24 g l −1 cyclodecanone after 24 h in reactions running at
substrate concentrations of 100 mM. The reactions were conducted with addition
+
of the oxidized cofactor form (NADP ), which was first reduced to NADPH by ADH-
catalyzed oxidation of 2-propanol to acetone (Scheme 5.25). This reaction concept
employing the P450 BM3 mutant 19A12 and L. kefir ADH revealed excellent TTNs
for cyclooctanone (11 641) and cyclohexanone (6531) formations, whereas the TTN
for cyclodecanone was lower (645).
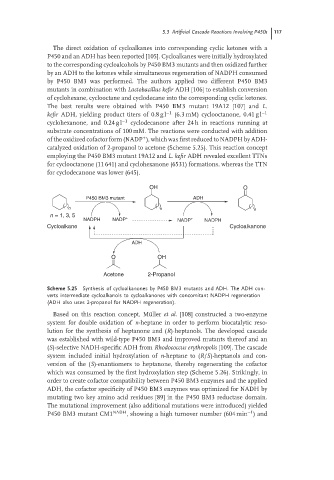
OH O
P450 BM3 mutant ADH
n n n
n = 1, 3, 5
NADPH NADP + NADP + NADPH
Cycloalkane Cycloalkanone
ADH
O OH
Acetone 2-Propanol
Scheme 5.25 Synthesis of cycloalkanones by P450 BM3 mutants and ADH. The ADH con-
verts intermediate cycloalkanols to cycloalkanones with concomitant NADPH regeneration
(ADH also uses 2-propanol for NADPH regeneration).
Based on this reaction concept, M¨ uller et al. [108] constructed a two-enzyme
system for double oxidation of n-heptane in order to perform biocatalytic reso-
lution for the synthesis of heptanone and (R)-heptanols. The developed cascade
was established with wild-type P450 BM3 and improved mutants thereof and an
(S)-selective NADH-specific ADH from Rhodococcus erythropolis [109]. The cascade
system included initial hydroxylation of n-heptane to (R/S)-heptanols and con-
version of the (S)-enantiomers to heptanone, thereby regenerating the cofactor
which was consumed by the first hydroxylation step (Scheme 5.26). Strikingly, in
order to create cofactor compatibility between P450 BM3 enzymes and the applied
ADH, the cofactor specificity of P450 BM3 enzymes was optimized for NADH by
mutating two key amino acid residues [89] in the P450 BM3 reductase domain.
The mutational improvement (also additional mutations were introduced) yielded
−1
P450 BM3 mutant CM1 NADH , showing a high turnover number (604 min )and