Page 139 - Cascade_Biocatalysis_Integrating_Stereoselective_and_Environmentally_Friendly_Reactions
P. 139
5.3 Artificial Cascade Reactions Involving P450s 115
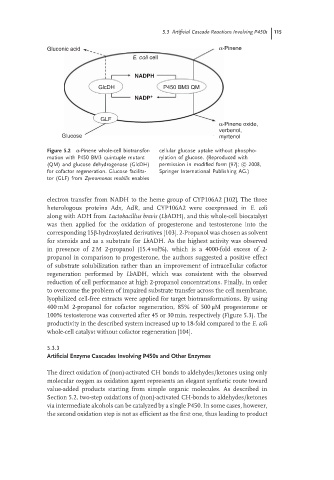
Gluconic acid α-Pinene
E. coli cell
NADPH
GlcDH P450 BM3 QM
NADP +
GLF
α-Pinene oxide,
verbenol,
Glucose myrtenol
Figure 5.2 α-Pinene whole-cell biotransfor- cellular glucose uptake without phospho-
mation with P450 BM3 quintuple mutant rylation of glucose. (Reproduced with
(QM) and glucose dehydrogenase (GlcDH) permission in modified form [97]; c 2008,
for cofactor regeneration. Glucose facilita- Springer International Publishing AG.)
tor (GLF) from Zymomonas mobilis enables
electron transfer from NADH to the heme group of CYP106A2 [102]. The three
heterologous proteins Adx, AdR, and CYP106A2 were coexpressed in E. coli
along with ADH from Lactobacillus brevis (LbADH), and this whole-cell biocatalyst
was then applied for the oxidation of progesterone and testosterone into the
corresponding 15β-hydroxylated derivatives [103]. 2-Propanol was chosen as solvent
for steroids and as a substrate for LbADH. As the highest activity was observed
in presence of 2 M 2-propanol (15.4 vol%), which is a 4000-fold excess of 2-
propanol in comparison to progesterone, the authors suggested a positive effect
of substrate solubilization rather than an improvement of intracellular cofactor
regeneration performed by LbADH, which was consistent with the observed
reduction of cell performance at high 2-propanol concentrations. Finally, in order
to overcome the problem of impaired substrate transfer across the cell membrane,
lyophilized cell-free extracts were applied for target biotransformations. By using
400 mM 2-propanol for cofactor regeneration, 85% of 500 μM progesterone or
100% testosterone was converted after 45 or 30 min, respectively (Figure 5.3). The
productivity in the described system increased up to 18-fold compared to the E. coli
whole-cell catalyst without cofactor regeneration [104].
5.3.3
Artificial Enzyme Cascades Involving P450s and Other Enzymes
The direct oxidation of (non)-activated CH bonds to aldehydes/ketones using only
molecular oxygen as oxidation agent represents an elegant synthetic route toward
value-added products starting from simple organic molecules. As described in
Section 5.2, two-step oxidations of (non)-activated CH-bonds to aldehydes/ketones
via intermediate alcohols can be catalyzed by a single P450. In some cases, however,
the second oxidation step is not as efficient as the first one, thus leading to product