Page 135 - Cascade_Biocatalysis_Integrating_Stereoselective_and_Environmentally_Friendly_Reactions
P. 135
5.3 Artificial Cascade Reactions Involving P450s 111
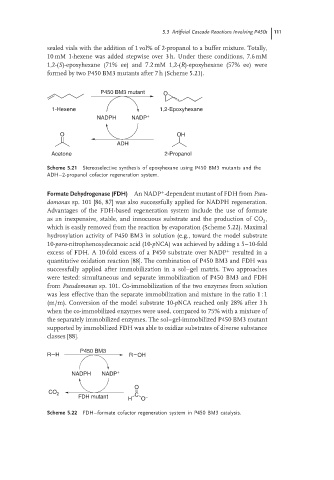
sealed vials with the addition of 1 vol% of 2-propanol to a buffer mixture. Totally,
10 mM 1-hexene was added stepwise over 3 h. Under these conditions, 7.6 mM
1,2-(S)-epoxyhexane (71% ee) and 7.2 mM 1,2-(R)-epoxyhexane (57% ee) were
formed by two P450 BM3 mutants after 7 h (Scheme 5.21).
P450 BM3 mutant O
1-Hexene 1,2-Epoxyhexane
NADPH NADP +
O OH
ADH
Acetone 2-Propanol
Scheme 5.21 Stereoselective synthesis of epoxyhexane using P450 BM3 mutants and the
ADH–2-propanol cofactor regeneration system.
+
Formate Dehydrogenase (FDH) An NADP -dependent mutant of FDH from Pseu-
domonas sp. 101 [86, 87] was also successfully applied for NADPH regeneration.
Advantages of the FDH-based regeneration system include the use of formate
as an inexpensive, stable, and innocuous substrate and the production of CO ,
2
which is easily removed from the reaction by evaporation (Scheme 5.22). Maximal
hydroxylation activity of P450 BM3 in solution (e.g., toward the model substrate
10-para-nitrophenoxydecanoic acid (10-pNCA) was achieved by adding a 5–10-fold
+
excess of FDH. A 10-fold excess of a P450 substrate over NADP resulted in a
quantitative oxidation reaction [88]. The combination of P450 BM3 and FDH was
successfully applied after immobilization in a sol–gel matrix. Two approaches
were tested: simultaneous and separate immobilization of P450 BM3 and FDH
from Pseudomonas sp. 101. Co-immobilization of the two enzymes from solution
was less effective than the separate immobilization and mixture in the ratio 1 : 1
(m/m). Conversion of the model substrate 10-pNCA reached only 28% after 3 h
when the co-immobilized enzymes were used, compared to 75% with a mixture of
the separately immobilized enzymes. The sol–gel-immobilized P450 BM3 mutant
supported by immobilized FDH was able to oxidize substrates of diverse substance
classes [88].
P450 BM3
RH ROH
NADPH NADP +
O
CO 2
FDH mutant H C O −
Scheme 5.22 FDH–formate cofactor regeneration system in P450 BM3 catalysis.