Page 133 - Cascade_Biocatalysis_Integrating_Stereoselective_and_Environmentally_Friendly_Reactions
P. 133
5.3 Artificial Cascade Reactions Involving P450s 109
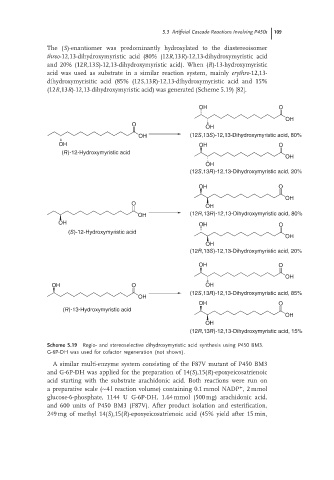
The (S)-enantiomer was predominantly hydroxylated to the diastereoisomer
threo-12,13-dihydroxymyristic acid (80% (12R,13R)-12,13-dihydroxymyristic acid
and 20% (12R,13S)-12,13-dihydroxymyristic acid). When (R)-13-hydroxymyristic
acid was used as substrate in a similar reaction system, mainly erythro-12,13-
dihydroxymyrisitic acid (85% (12S,13R)-12,13-dihydroxymyristic acid and 15%
(12R,13R)-12,13-dihydroxymyristic acid) was generated (Scheme 5.19) [82].
OH O
OH
O OH
OH (12S,13S)-12,13-Dihydroxymyristic acid, 80%
OH OH O
(R)-12-Hydroxymyristic acid
OH
OH
(12S,13R)-12,13-Dihydroxymyristic acid, 20%
OH O
OH
O
OH
OH (12R,13R)-12,13-Dihydroxymyristic acid, 80%
OH OH O
(S)-12-Hydroxymyristic acid
OH
OH
(12R,13S)-12,13-Dihydroxymyristic acid, 20%
OH O
OH
OH O OH
(12S,13R)-12,13-Dihydroxymyristic acid, 85%
OH
OH O
(R)-13-Hydroxymyristic acid
OH
OH
(12R,13R)-12,13-Dihydroxymyristic acid, 15%
Scheme 5.19 Regio- and stereoselective dihydroxymyristic acid synthesis using P450 BM3.
G-6P-DH was used for cofactor regeneration (not shown).
A similar multi-enzyme system consisting of the F87V mutant of P450 BM3
and G-6P-DH was applied for the preparation of 14(S),15(R)-epoxyeicosatrienoic
acid starting with the substrate arachidonic acid. Both reactions were run on
+
a preparative scale (∼4 l reaction volume) containing 0.1 mmol NADP , 2 mmol
glucose-6-phosphate, 1144 U G-6P-DH, 1.64 mmol (500 mg) arachidonic acid,
and 600 units of P450 BM3 (F87V). After product isolation and esterification,
249 mg of methyl 14(S),15(R)-epoxyeicosatrienoic acid (45% yield after 15 min,