Page 128 - Cascade_Biocatalysis_Integrating_Stereoselective_and_Environmentally_Friendly_Reactions
P. 128
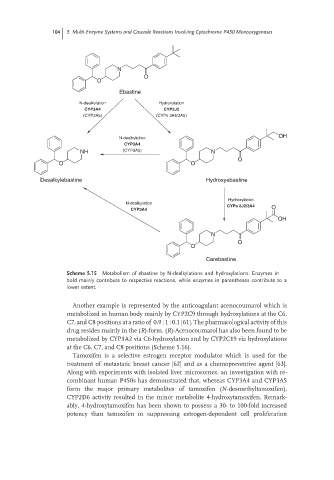
104 5 Multi-Enzyme Systems and Cascade Reactions Involving Cytochrome P450 Monooxygenases
N
O
O
Ebastine
N-dealkylation Hydroxylation
CYP3A4 CYP2J2
(CYP3A5) (CYPs 3A4/3A5)
OH
N-dealkylation
CYP3A4
NH (CYP3A5) N
O
O O
Desalkylebastine Hydroxyebastine
Hydroxylation
N-dealkylation
CYPs 2J2/3A4 O
CYP3A4
OH
N
O
O
Carebastine
Scheme 5.15 Metabolism of ebastine by N-dealkylations and hydroxylations. Enzymes in
bold mainly contribute to respective reactions, while enzymes in parentheses contribute to a
lower extent.
Another example is represented by the anticoagulant acenocoumarol which is
metabolized in human body mainly by CYP2C9 through hydroxylations at the C6,
C7, and C8 positions at a ratio of 0.9 : 1 : 0.1 [61]. The pharmacological activity of this
drug resides mainly in the (R)-form. (R)-Acenocoumarol has also been found to be
metabolized by CYP1A2 via C6-hydroxylation and by CYP2C19 via hydroxylations
at the C6, C7, and C8 positions (Scheme 5.16).
Tamoxifen is a selective estrogen receptor modulator which is used for the
treatment of metastatic breast cancer [62] and as a chemopreventive agent [63].
Along with experiments with isolated liver microsomes, an investigation with re-
combinant human P450s has demonstrated that, whereas CYP3A4 and CYP3A5
form the major primary metabolites of tamoxifen (N-desmethyltamoxifen),
CYP2D6 activity resulted in the minor metabolite 4-hydroxytamoxifen. Remark-
ably, 4-hydroxytamoxifen has been shown to possess a 30- to 100-fold increased
potency than tamoxifen in suppressing estrogen-dependent cell proliferation