Page 123 - Cascade_Biocatalysis_Integrating_Stereoselective_and_Environmentally_Friendly_Reactions
P. 123
5.2 Physiological Cascade Reactions Involving P450s 99
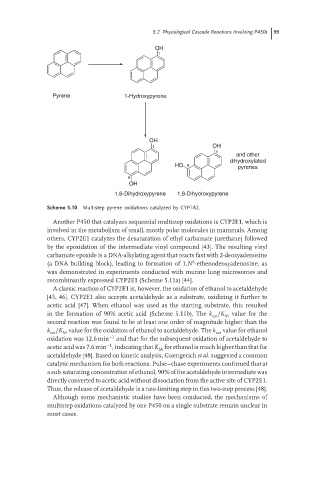
OH
1
Pyrene 1-Hydroxypyrene
OH
1 OH
1
and other
dihydroxylated
HO 8 pyrenes
6
OH
1,6-Dihydroxypyrene 1,8-Dihydroxypyrene
Scheme 5.10 Multistep pyrene oxidations catalyzed by CYP1A2.
Another P450 that catalyzes sequential multistep oxidations is CYP2E1, which is
involved in the metabolism of small, mostly polar molecules in mammals. Among
others, CYP2E1 catalyzes the desaturation of ethyl carbamate (urethane) followed
by the epoxidation of the intermediate vinyl compound [43]. The resulting vinyl
carbamate epoxide is a DNA-alkylating agent that reacts fast with 2-deoxyadenosine
6
(a DNA building block), leading to formation of 1,N -ethenodeoxyadenosine, as
was demonstrated in experiments conducted with murine lung microsomes and
recombinantly expressed CYP2E1 (Scheme 5.11a) [44].
A classic reaction of CYP2E1 is, however, the oxidation of ethanol to acetaldehyde
[45, 46]. CYP2E1 also accepts acetaldehyde as a substrate, oxidizing it further to
acetic acid [47]. When ethanol was used as the starting substrate, this resulted
in the formation of 90% acetic acid (Scheme 5.11b). The k /K value for the
cat M
second reaction was found to be at least one order of magnitude higher than the
k /K value for the oxidation of ethanol to acetaldehyde. The k value for ethanol
cat M cat
oxidation was 12.6 min −1 and that for the subsequent oxidation of acetaldehyde to
−1
acetic acid was 7.6 min , indicating that K for ethanol is much higher than that for
M
acetaldehyde [48]. Based on kinetic analysis, Guengerich et al. suggested a common
catalytic mechanism for both reactions. Pulse–chase experiments confirmed that at
a sub-saturating concentration of ethanol, 90% of the acetaldehyde intermediate was
directly converted to acetic acid without dissociation from the active site of CYP2E1.
Thus, the release of acetaldehyde is a rate-limiting step in this two-step process [48].
Although some mechanistic studies have been conducted, the mechanisms of
multistep oxidations catalyzed by one P450 on a single substrate remain unclear in
most cases.