Page 121 - Cascade_Biocatalysis_Integrating_Stereoselective_and_Environmentally_Friendly_Reactions
P. 121
5.2 Physiological Cascade Reactions Involving P450s 97
14
32
OH
HO HO
Lanosterol
14
32 O
HO
HCOOH
14
HO
4,4-Dimethyl-5α-cholesta-8,14,24-diene-3β-ol
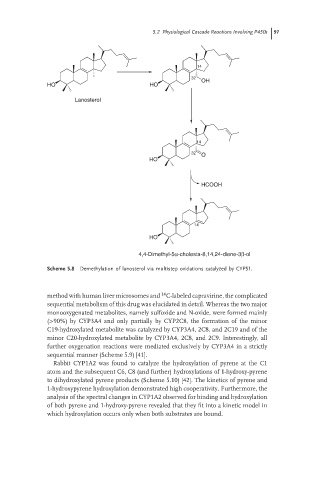
Scheme 5.8 Demethylation of lanosterol via multistep oxidations catalyzed by CYP51.
14
method with human liver microsomes and C-labeled capravirine, the complicated
sequential metabolism of this drug was elucidated in detail. Whereas the two major
monooxygenated metabolites, namely sulfoxide and N-oxide, were formed mainly
(>90%) by CYP3A4 and only partially by CYP2C8, the formation of the minor
C19-hydroxylated metabolite was catalyzed by CYP3A4, 2C8, and 2C19 and of the
minor C20-hydroxylated metabolite by CYP3A4, 2C8, and 2C9. Interestingly, all
further oxygenation reactions were mediated exclusively by CYP3A4 in a strictly
sequential manner (Scheme 5.9) [41].
Rabbit CYP1A2 was found to catalyze the hydroxylation of pyrene at the C1
atom and the subsequent C6, C8 (and further) hydroxylations of 1-hydroxy-pyrene
to dihydroxylated pyrene products (Scheme 5.10) [42]. The kinetics of pyrene and
1-hydroxypyrene hydroxylation demonstrated high cooperativity. Furthermore, the
analysis of the spectral changes in CYP1A2 observed for binding and hydroxylation
of both pyrene and 1-hydroxy-pyrene revealed that they fit into a kinetic model in
which hydroxylation occurs only when both substrates are bound.