Page 117 - Cascade_Biocatalysis_Integrating_Stereoselective_and_Environmentally_Friendly_Reactions
P. 117
5.2 Physiological Cascade Reactions Involving P450s 93
22 HO 22
20
H H OH H OH
H H H
H H H H H H
HO HO HO
Cholesterol 22(R)-Hydroxycholesterol 20α,22(R)-Dihydroxycholesterol
O
H
H H
HO
Pregnenolone
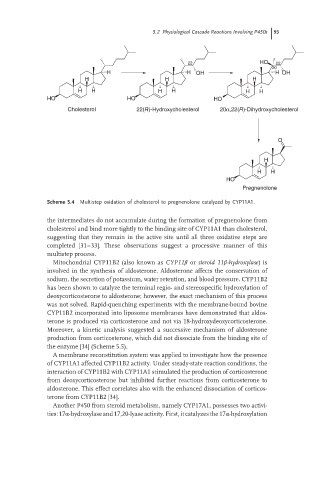
Scheme 5.4 Multistep oxidation of cholesterol to pregnenolone catalyzed by CYP11A1.
the intermediates do not accumulate during the formation of pregnenolone from
cholesterol and bind more tightly to the binding site of CYP11A1 than cholesterol,
suggesting that they remain in the active site until all three oxidative steps are
completed [31–33]. These observations suggest a processive manner of this
multistep process.
Mitochondrial CYP11B2 (also known as CYP11 or steroid 11 -hydroxylase)is
involved in the synthesis of aldosterone. Aldosterone affects the conservation of
sodium, the secretion of potassium, water retention, and blood pressure. CYP11B2
has been shown to catalyze the terminal regio- and stereospecific hydroxylation of
deoxycorticosterone to aldosterone; however, the exact mechanism of this process
was not solved. Rapid-quenching experiments with the membrane-bound bovine
CYP11B2 incorporated into liposome membranes have demonstrated that aldos-
terone is produced via corticosterone and not via 18-hydroxydeoxycorticosterone.
Moreover, a kinetic analysis suggested a successive mechanism of aldosterone
production from corticosterone, which did not dissociate from the binding site of
the enzyme [34] (Scheme 5.5).
A membrane reconstitution system was applied to investigate how the presence
of CYP11A1 affected CYP11B2 activity. Under steady-state reaction conditions, the
interaction of CYP11B2 with CYP11A1 stimulated the production of corticosterone
from deoxycorticosterone but inhibited further reactions from corticosterone to
aldosterone. This effect correlates also with the enhanced dissociation of corticos-
terone from CYP11B2 [34].
Another P450 from steroid metabolism, namely CYP17A1, possesses two activi-
ties: 17α-hydroxylase and 17,20-lyase activity. First, it catalyzes the 17α-hydroxylation