Page 114 - Cascade_Biocatalysis_Integrating_Stereoselective_and_Environmentally_Friendly_Reactions
P. 114
90 5 Multi-Enzyme Systems and Cascade Reactions Involving Cytochrome P450 Monooxygenases
substrate, the hydroxylated product is released (7), and the enzyme returns to the
initial water-bound state (1). This catalytic cycle requires the timely delivery of
two electrons to the heme iron, which are derived from NADH or NADPH and
transferred to the heme iron of a P450 via separate redox partner proteins (see
below).
Under certain conditions, reduction of heme iron does not result in substrate
oxidation. Such events are referred to as uncoupling pathways or shunt pathways.
Three uncoupling pathways can be distinguished in the P450 catalytic cycle:
autoxidation shunt, oxidase shunt, and peroxide shunt (Scheme 5.2). For bio-
catalytic applications, it is particularly important to take into account that, upon
uncoupling, reducing equivalents derived from NAD(P)H are consumed with-
out product formation. On the other hand, the ‘‘peroxide shunt’’ can be forced
into a productive direction through the addition of hydrogen peroxide or organic
peroxides [15, 16].
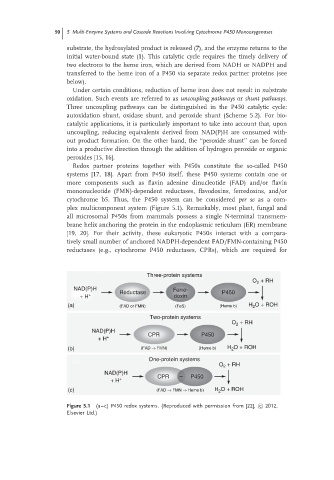
Redox partner proteins together with P450s constitute the so-called P450
systems [17, 18]. Apart from P450 itself, these P450 systems contain one or
more components such as flavin adenine dinucleotide (FAD) and/or flavin
mononucleotide (FMN)-dependent reductases, flavodoxins, ferredoxins, and/or
cytochrome b5. Thus, the P450 system can be considered per se as a com-
plex multicomponent system (Figure 5.1). Remarkably, most plant, fungal and
all microsomal P450s from mammals possess a single N-terminal transmem-
brane helix anchoring the protein in the endoplasmic reticulum (ER) membrane
[19, 20]. For their activity, these eukaryotic P450s interact with a compara-
tively small number of anchored NADPH-dependent FAD/FMN-containing P450
reductases (e.g., cytochrome P450 reductases, CPRs), which are required for
Three-protein systems
O 2 + RH
NAD(P)H Ferre-
Reductase P450
+ H + doxin
(a) (FAD or FMN) (FeS) (Heme b) H 2 O + ROH
Two-protein systems
O + RH
2
NAD(P)H CPR P450
+ H +
(b) (FAD → FMN) (Heme b) H O + ROH
2
One-protein systems
O + RH
2
NAD(P)H
+ H + CPR P450
(c) (FAD → FMN → Heme b) H O + ROH
2
Figure 5.1 (a–c) P450 redox systems. (Reproduced with permission from [22], c 2012,
Elsevier Ltd.)