Page 118 - Cascade_Biocatalysis_Integrating_Stereoselective_and_Environmentally_Friendly_Reactions
P. 118
94 5 Multi-Enzyme Systems and Cascade Reactions Involving Cytochrome P450 Monooxygenases
O OH
HO
11
H
H H
O
O OH O OH
Corticosterone HO
18
HO
11
H H
O OH
H H HO H H
O 18 O
Deoxycorticosterone H 18-Hydoxycorticosterone
H H
O
18-Hydroxydeoxycorticosterone
O OH
O 18
HO
11
H
H H
O
Aldosterone
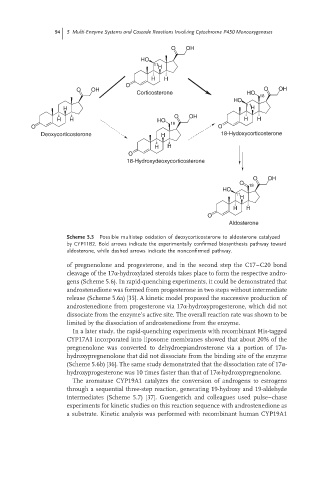
Scheme 5.5 Possible multistep oxidation of deoxycorticosterone to aldosterone catalyzed
by CYP11B2. Bold arrows indicate the experimentally confirmed biosynthesis pathway toward
aldosterone, while dashed arrows indicate the nonconfirmed pathway.
of pregnenolone and progesterone, and in the second step the C17–C20 bond
cleavage of the 17α-hydroxylated steroids takes place to form the respective andro-
gens (Scheme 5.6). In rapid-quenching experiments, it could be demonstrated that
androstenedione was formed from progesterone in two steps without intermediate
release (Scheme 5.6a) [35]. A kinetic model proposed the successive production of
androstenedione from progesterone via 17α-hydroxyprogesterone, which did not
dissociate from the enzyme’s active site. The overall reaction rate was shown to be
limited by the dissociation of androstenedione from the enzyme.
In a later study, the rapid-quenching experiments with recombinant His-tagged
CYP17A1 incorporated into liposome membranes showed that about 20% of the
pregnenolone was converted to dehydroepiandrosterone via a portion of 17α-
hydroxypregnenolone that did not dissociate from the binding site of the enzyme
(Scheme 5.6b) [36]. The same study demonstrated that the dissociation rate of 17α-
hydroxyprogesterone was 10 times faster than that of 17α-hydroxypregnenolone.
The aromatase CYP19A1 catalyzes the conversion of androgens to estrogens
through a sequential three-step reaction, generating 19-hydroxy and 19-aldehyde
intermediates (Scheme 5.7) [37]. Guengerich and colleagues used pulse–chase
experiments for kinetic studies on this reaction sequence with androstenedione as
a substrate. Kinetic analysis was performed with recombinant human CYP19A1