Page 120 - Cascade_Biocatalysis_Integrating_Stereoselective_and_Environmentally_Friendly_Reactions
P. 120
96 5 Multi-Enzyme Systems and Cascade Reactions Involving Cytochrome P450 Monooxygenases
R R
HO
H 19 H
H H H H
O O
Testosterone (R: H, β-OH)
Androstenedione (R: O)
R
O
19 H
H H
O
O
H OH
R
H
H H
HO
17β-Estradiol (R: H, β-OH)
Estrone (R: O)
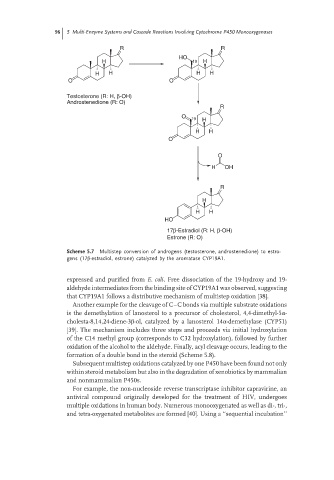
Scheme 5.7 Multistep conversion of androgens (testosterone, androstenedione) to estro-
gens (17β-estradiol, estrone) catalyzed by the aromatase CYP19A1.
expressed and purified from E. coli. Free dissociation of the 19-hydroxy and 19-
aldehyde intermediates from the binding site of CYP19A1 was observed, suggesting
that CYP19A1 follows a distributive mechanism of multistep oxidation [38].
Another example for the cleavage of C–C bonds via multiple substrate oxidations
is the demethylation of lanosterol to a precursor of cholesterol, 4,4-dimethyl-5α-
cholesta-8,14,24-diene-3β-ol, catalyzed by a lanosterol 14α-demethylase (CYP51)
[39]. The mechanism includes three steps and proceeds via initial hydroxylation
of the C14 methyl group (corresponds to C32 hydroxylation), followed by further
oxidation of the alcohol to the aldehyde. Finally, acyl cleavage occurs, leading to the
formation of a double bond in the steroid (Scheme 5.8).
Subsequent multistep oxidations catalyzed by one P450 have been found not only
within steroid metabolism but also in the degradation of xenobiotics by mammalian
and nonmammalian P450s.
For example, the non-nucleoside reverse transcriptase inhibitor capravirine, an
antiviral compound originally developed for the treatment of HIV, undergoes
multiple oxidations in human body. Numerous monooxygenated as well as di-, tri-,
and tetra-oxygenated metabolites are formed [40]. Using a ‘‘sequential incubation’’