Page 124 - Cascade_Biocatalysis_Integrating_Stereoselective_and_Environmentally_Friendly_Reactions
P. 124
100 5 Multi-Enzyme Systems and Cascade Reactions Involving Cytochrome P450 Monooxygenases
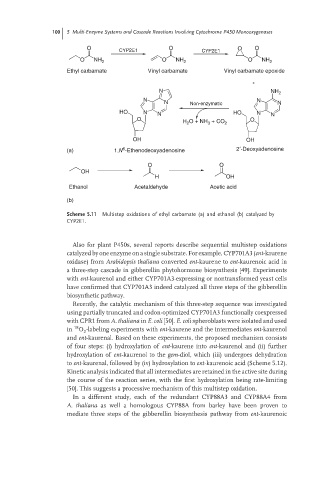
O O O O
CYP2E1 CYP2E1
O NH 2 O NH 2 O NH 2
Ethyl carbamate Vinyl carbamate Vinyl carbamate epoxide
N NH 2
N N
N Non-enzymatic N
HO N N HO N N
O O
H 2 O + NH 3 + CO 2
OH OH
6
(a) 1,N -Ethenodeoxyadenosine 2′-Deoxyadenosine
O O
OH
H OH
Ethanol Acetaldehyde Acetic acid
(b)
Scheme 5.11 Multistep oxidations of ethyl carbamate (a) and ethanol (b) catalyzed by
CYP2E1.
Also for plant P450s, several reports describe sequential multistep oxidations
catalyzed by one enzyme on a single substrate. For example, CYP701A3 (ent-kaurene
oxidase) from Arabidopsis thaliana converted ent-kaurene to ent-kaurenoic acid in
a three-step cascade in gibberellin phytohormone biosynthesis [49]. Experiments
with ent-kaurenol and either CYP701A3-expressing or nontransformed yeast cells
have confirmed that CYP701A3 indeed catalyzed all three steps of the gibberellin
biosynthetic pathway.
Recently, the catalytic mechanism of this three-step sequence was investigated
using partially truncated and codon-optimized CYP701A3 functionally coexpressed
with CPR1 from A. thaliana in E. coli [50]. E. coli spheroblasts were isolated and used
in 18 O -labeling experiments with ent-kaurene and the intermediates ent-kaurenol
2
and ent-kaurenal. Based on these experiments, the proposed mechanism consists
of four steps: (i) hydroxylation of ent-kaurene into ent-kaurenol and (ii) further
hydroxylation of ent-kaurenol to the gem-diol, which (iii) undergoes dehydration
to ent-kaurenal, followed by (iv) hydroxylation to ent-kaurenoic acid (Scheme 5.12).
Kinetic analysis indicated that all intermediates are retained in the active site during
the course of the reaction series, with the first hydroxylation being rate-limiting
[50]. This suggests a processive mechanism of this multistep oxidation.
In a different study, each of the redundant CYP88A3 and CYP88A4 from
A. thaliana as well a homologous CYP88A from barley have been proven to
mediate three steps of the gibberellin biosynthesis pathway from ent-kaurenoic