Page 126 - Cascade_Biocatalysis_Integrating_Stereoselective_and_Environmentally_Friendly_Reactions
P. 126
102 5 Multi-Enzyme Systems and Cascade Reactions Involving Cytochrome P450 Monooxygenases
5.2.2
Multistep Oxidations Catalyzed by Multiple P450s
As mentioned in the previous section, endogenous compounds such as steroids
and prostaglandins, as well as exogenous xenobiotic compounds such as drugs or
environmental pollutants, undergo multistep degradation processing in the human
body. Five human hepatic P450s, namely CYPs 1A2, 2C9, 2C19, 2D6, and 3A4,
are involved in almost 95% of the P450-mediated drug metabolism (and in about
75% of total drug metabolism) [52, 53]. Among them, CYP3A4 is suggested to
metabolize about half of all administered drugs.
There are obviously several scenarios for the action mode of hepatic P450s.
In many cases, different hepatic P450s belonging either to one or several
subfamilies perform various oxygenation reactions at different positions of a single
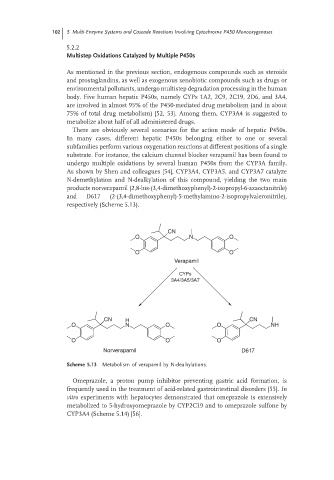
substrate. For instance, the calcium channel blocker verapamil has been found to
undergo multiple oxidations by several human P450s from the CYP3A family.
As shown by Shen and colleagues [54], CYP3A4, CYP3A5, and CYP3A7 catalyze
N-demethylation and N-dealkylation of this compound, yielding the two main
products norverapamil (2,8-bis-(3,4-dimethoxyphenyl)-2-isopropyl-6-azaoctanitrile)
and D617 (2-(3,4-dimethoxyphenyl)-5-methylamino-2-isopropylvaleronitrile),
respectively (Scheme 5.13).
CN
O N O
O O
Verapamil
CYPs
3A4/3A5/3A7
CN H CN
O N O O NH
O O O
Norverapamil D617
Scheme 5.13 Metabolism of verapamil by N-dealkylations.
Omeprazole, a proton pump inhibitor preventing gastric acid formation, is
frequently used in the treatment of acid-related gastrointestinal disorders [55]. In
vitro experiments with hepatocytes demonstrated that omeprazole is extensively
metabolized to 5-hydroxyomeprazole by CYP2C19 and to omeprazole sulfone by
CYP3A4 (Scheme 5.14) [56].