Page 131 - Cascade_Biocatalysis_Integrating_Stereoselective_and_Environmentally_Friendly_Reactions
P. 131
5.2 Physiological Cascade Reactions Involving P450s 107
[64, 65]. N-Desmethyl- and 4-hydroxy-tamoxifen were used to examine the secondary
metabolism of tamoxifen. N-Desmethyl-tamoxifen was predominantly biotrans-
formed to α-hydroxy-, N-desmethyl-, N-didesmethyl-, and 4-hydroxy-N-desmethyl-
tamoxifen (endoxifen). 4-Hydroxytamoxifen was converted to 3,4-dihydroxyta-
moxifen and endoxifen. Among all the mentioned reactions, only the oxidation
of N-desmethyl-tamoxifen to 4-hydroxyl-N-desmethyltamoxifen (endoxifen) was
exclusively catalyzed by CYP2D6. All other reactions of the secondary metabolism of
tamoxifen were catalyzed predominantly by the CYP3A enzymes (Scheme 5.17) [66].
In analogy to exogenous substances, endogenous compounds such as steroids
(see above), prostaglandins, and vitamins are metabolized by several P450s in
multiple steps. One example that is particularly worth mentioning is the metabolism
of vitamin D3. Vitamin D3 is a secosteroid involved in a wide variety of biological
functions in humans [67]. It shows no hormonal activity itself, but is converted into
its active form by several P450 enzymes.
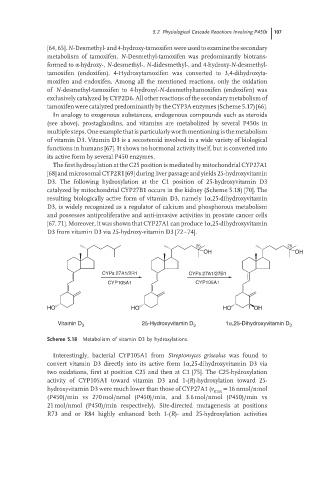
The first hydroxylation at the C25 position is mediated by mitochondrial CYP27A1
[68] and microsomal CYP2R1 [69] during liver passage and yields 25-hydroxyvitamin
D3. The following hydroxylation at the C1 position of 25-hydroxyvitamin D3
catalyzed by mitochondrial CYP27B1 occurs in the kidney (Scheme 5.18) [70]. The
resulting biologically active form of vitamin D3, namely 1α,25-dihydroxyvitamin
D3, is widely recognized as a regulator of calcium and phosphorous metabolism
and possesses antiproliferative and anti-invasive activities in prostate cancer cells
[67, 71]. Moreover, it was shown that CYP27A1 can produce 1α,25-dihydroxyvitamin
D3 from vitamin D3 via 25-hydroxy-vitamin D3 [72–74].
25 25
OH OH
CYPs 27A1/2R1 CYPs 27A1/27B1
CYP105A1 CYP105A1
HO HO HO 1 OH
Vitamin D 3 25-Hydroxyvitamin D 3 1α,25-Dihydroxyvitamin D 3
Scheme 5.18 Metabolism of vitamin D3 by hydroxylations.
Interestingly, bacterial CYP105A1 from Streptomyces griseolus was found to
convert vitamin D3 directly into its active form 1α,25-dihydroxyvitamin D3 via
two oxidations, first at position C25 and then at C1 [75]. The C25-hydroxylation
activity of CYP105A1 toward vitamin D3 and 1-(R)-hydroxylation toward 25-
hydroxyvitamin D3 were much lower than those of CYP27A1 (v = 16 nmol/nmol
max
(P450)/min vs 270 mol/nmol (P450)/min, and 3.6 mol/nmol (P450)/min vs
21 mol/nmol (P450)/min respectively). Site-directed mutagenesis at positions
R73 and or R84 highly enhanced both 1-(R)- and 25-hydroxylation activities