Page 142 - Cascade_Biocatalysis_Integrating_Stereoselective_and_Environmentally_Friendly_Reactions
P. 142
118 5 Multi-Enzyme Systems and Cascade Reactions Involving Cytochrome P450 Monooxygenases
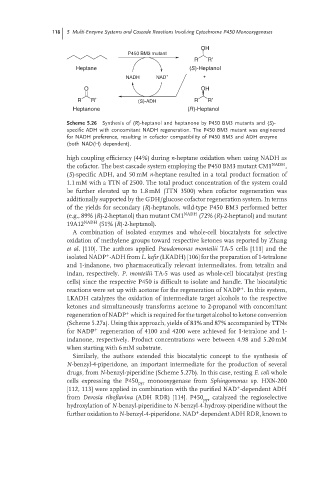
OH
P450 BM3 mutant
R R′
Heptane (S)-Heptanol
NADH NAD +
O OH
R R′ (S)-ADH R R′
Heptanone (R)-Heptanol
Scheme 5.26 Synthesis of (R)-heptanol and heptanone by P450 BM3 mutants and (S)-
specific ADH with concomitant NADH regeneration. The P450 BM3 mutant was engineered
for NADH preference, resulting in cofactor compatibility of P450 BM3 and ADH enzyme
(both NAD(H) dependent).
high coupling efficiency (44%) during n-heptane oxidation when using NADH as
the cofactor. The best cascade system employing the P450 BM3 mutant CM1 NADH ,
(S)-specific ADH, and 50 mM n-heptane resulted in a total product formation of
1.1 mM with a TTN of 2500. The total product concentration of the system could
be further elevated up to 1.8 mM (TTN 3500) when cofactor regeneration was
additionally supported by the GDH/glucose cofactor regeneration system. In terms
of the yields for secondary (R)-heptanols, wild-type P450 BM3 performed better
(e.g., 89% (R)-2-heptanol) than mutant CM1 NADH (72% (R)-2-heptanol) and mutant
19A12 NADH (51% (R)-2-heptanol).
A combination of isolated enzymes and whole-cell biocatalysts for selective
oxidation of methylene groups toward respective ketones was reported by Zhang
et al. [110]. The authors applied Pseudomonas monteilii TA-5 cells [111] and the
+
isolated NADP -ADH from L. kefir (LKADH) [106] for the preparation of 1-tetralone
and 1-indanone, two pharmaceutically relevant intermediates, from tetralin and
indan, respectively. P. monteilii TA-5 was used as whole-cell biocatalyst (resting
cells) since the respective P450 is difficult to isolate and handle. The biocatalytic
+
reactions were set up with acetone for the regeneration of NADP . In this system,
LKADH catalyzes the oxidation of intermediate target alcohols to the respective
ketones and simultaneously transforms acetone to 2-propanol with concomitant
+
regeneration of NADP which is required for the target alcohol to ketone conversion
(Scheme 5.27a). Using this approach, yields of 83% and 87% accompanied by TTNs
+
for NADP regeneration of 4100 and 4200 were achieved for 1-tetralone and 1-
indanone, respectively. Product concentrations were between 4.98 and 5.20 mM
when starting with 6 mM substrate.
Similarly, the authors extended this biocatalytic concept to the synthesis of
N-benzyl-4-piperidone, an important intermediate for the production of several
drugs, from N-benzyl-piperidine (Scheme 5.27b). In this case, resting E. coli whole
cells expressing the P450 pyr monooxygenase from Sphingomonas sp. HXN-200
+
[112, 113] were applied in combination with the purified NAD -dependent ADH
from Devosia riboflavina (ADH RDR) [114]. P450 pyr catalyzed the regioselective
hydroxylation of N-benzyl-piperidine to N-benzyl-4-hydroxy-piperidine without the
+
further oxidation to N-benzyl-4-piperidone. NAD -dependent ADH RDR, known to