Page 204 - Cascade_Biocatalysis_Integrating_Stereoselective_and_Environmentally_Friendly_Reactions
P. 204
180 8 Racemizable Acyl Donors for Enzymatic Dynamic Kinetic Resolution
it is very important to understand the racemization conditions of various classes of
compounds, not only in order to avoid this phenomenon for the product, but on
the contrary to exploit it for the substrate by setting up a reacting system suitable
for carrying out an effective DKR.
The aim of this overview is to focus the attention toward the racemization
conditions of two specific acyl donors commonly used for enzyme-catalyzed
synthesis, namely the oxoesters and the thioesters. The former are often the
substrates of choice because their mild reactivity (in contrast to anhydrides, for
instance) limits their susceptibility to parasitic reactions such as spontaneous
transacylation or condensation reactions, while at the same time they are easily
transformed by enzymes. Thioesters, though being ‘‘activated’’ substrates from a
thermodynamic point of view, possess a peculiar relative kinetic stability against
spontaneous hydrolysis [10], which permits their use in biocatalysis; moreover, they
are far more prone to base-catalyzed racemization as compared to their oxygenated
analogs [11]. This combination of features makes them attractive as candidates to
be employed in DKRs as alternatives to oxoesters, and examples will be discussed
to support this concept.
8.2
The Tools
8.2.1
The Enzymes
A recent report by the Swiss Industrial Biocatalysis Consortium about the state-of-
the-art in biocatalysis shows that hydrolases (EC 3) are, at least from the academic
point of view, still the most studied and applied biocatalysts [12], despite having
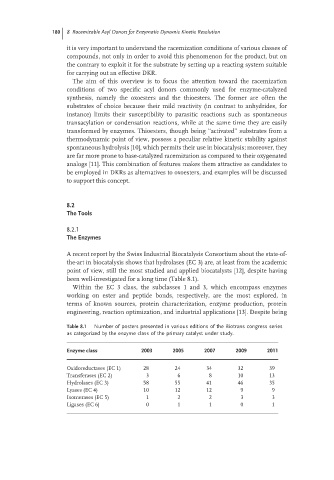
been well-investigated for a long time (Table 8.1).
Within the EC 3 class, the subclasses 1 and 3, which encompass enzymes
working on ester and peptide bonds, respectively, are the most explored, in
terms of known sources, protein characterization, enzyme production, protein
engineering, reaction optimization, and industrial applications [13]. Despite being
Table 8.1 Number of posters presented in various editions of the Biotrans congress series
as categorized by the enzyme class of the primary catalyst under study.
Enzyme class 2003 2005 2007 2009 2011
Oxidoreductases (EC 1) 28 24 34 32 39
Transferases (EC 2) 3 6 8 10 13
Hydrolases (EC 3) 58 55 41 46 35
Lyases (EC 4) 10 12 12 9 9
Isomerases (EC 5) 1 2 2 3 3
Ligases (EC 6) 0 1 1 0 1