Page 208 - Cascade_Biocatalysis_Integrating_Stereoselective_and_Environmentally_Friendly_Reactions
P. 208
184 8 Racemizable Acyl Donors for Enzymatic Dynamic Kinetic Resolution
(Scheme 8.2), the authors were, in fact, able to correlate the rate of formation of
the deuterated compound with the actual pK of the proton [11].
a
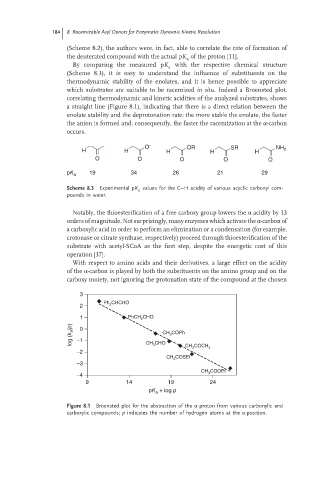
By comparing the measured pK with the respective chemical structure
a
(Scheme 8.3), it is easy to understand the influence of substituents on the
thermodynamic stability of the enolates, and it is hence possible to appreciate
which substrates are suitable to be racemized in situ. Indeed a Broensted plot,
correlating thermodynamic and kinetic acidities of the analyzed substrates, shows
a straight line (Figure 8.1), indicating that there is a direct relation between the
enolate stability and the deprotonation rate: the more stable the enolate, the faster
the anion is formed and, consequently, the faster the racemization at the α-carbon
occurs.
O − OR SR
H H H H H NH 2
O O O O O
19 34 26 21 29
pK a
Scheme 8.3 Experimental pK values for the C–H acidity of various acyclic carbonyl com-
a
pounds in water.
Notably, the thioesterification of a free carboxy group lowers the α acidity by 13
orders of magnitude. Not surprisingly, many enzymes which activate the α-carbon of
a carboxylic acid in order to perform an elimination or a condensation (for example,
crotonase or citrate synthase, respectively) proceed through thioesterification of the
substrate with acetyl-SCoA as the first step, despite the energetic cost of this
operation [37].
With respect to amino acids and their derivatives, a large effect on the acidity
of the α-carbon is played by both the substituents on the amino group and on the
carboxy moiety, not ignoring the protonation state of the compound at the chosen
3
Ph CHCHO
2 2
1 PhCH 2 CHO
log (k a /p) −1 CH 3 COPh
0
CH 3 CHO
CH 3 COCH 3
−2
CH COSEt
3
−3
COOEt
CH 3
−4
9 14 19 24
pK a + log p
Figure 8.1 Broensted plot for the abstraction of the α proton from various carbonylic and
carboxylic compounds; p indicates the number of hydrogen atoms at the α-position.