Page 213 - Cascade_Biocatalysis_Integrating_Stereoselective_and_Environmentally_Friendly_Reactions
P. 213
8.3 Applications of DKR to Acyl Compounds 189
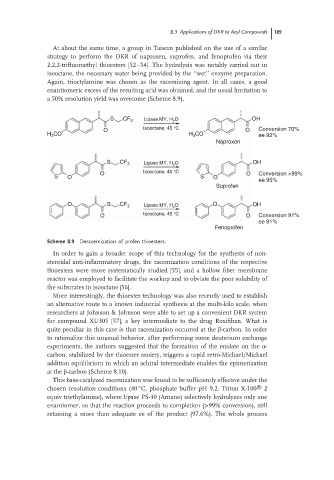
At about the same time, a group in Taiwan published on the use of a similar
strategy to perform the DKR of naproxen, suprofen, and fenoprofen via their
2,2,2-trifluoroethyl thioesters [52–54]. The hydrolysis was notably carried out in
isooctane, the necessary water being provided by the ‘‘wet’’ enzyme preparation.
Again, trioctylamine was chosen as the racemizing agent. In all cases, a good
enantiomeric excess of the resulting acid was obtained, and the usual limitation to
a 50% resolution yield was overcome (Scheme 8.9).
S CF 3 Lipase MY, H O OH
2
O Isooctane, 45 °C O Conversion 70%
H 3 CO H 3 CO ee 92%
Naproxen
S CF 3 Lipase MY, H 2 O OH
Isooctane, 45 °C
O O Conversion >99%
S O S O
ee 95%
Suprofen
O S CF 3 Lipase MY, H 2 O O OH
O Isooctane, 45 °C O Conversion 91%
ee 91%
Fenoprofen
Scheme 8.9 Deracemization of profen thioesters.
In order to gain a broader scope of this technology for the synthesis of non-
steroidal anti-inflammatory drugs, the racemization conditions of the respective
thioesters were more systematically studied [55], and a hollow fiber membrane
reactor was employed to facilitate the workup and to obviate the poor solubility of
the substrates in isooctane [56].
More interestingly, the thioester technology was also recently used to establish
an alternative route to a known industrial synthesis at the multi-kilo scale, when
researchers at Johnson & Johnson were able to set up a convenient DKR system
for compound XU305 [57], a key intermediate to the drug Roxifiban. What is
quite peculiar in this case is that racemization occurred at the β-carbon. In order
to rationalize this unusual behavior, after performing some deuterium exchange
experiments, the authors suggested that the formation of the enolate on the α-
carbon, stabilized by the thioester moiety, triggers a rapid retro-Michael/Michael
addition equilibrium in which an achiral intermediate enables the epimerization
at the β-carbon (Scheme 8.10).
This base-catalyzed racemization was found to be sufficiently effective under the
◦
chosen resolution conditions (40 C, phosphate buffer pH 9.2, Triton X-100 ® 2
equiv triethylamine), where lipase PS-30 (Amano) selectively hydrolyzes only one
enantiomer, so that the reaction proceeds to completion (>99% conversion), still
retaining a more than adequate ee of the product (97.6%). The whole process