Page 216 - Cascade_Biocatalysis_Integrating_Stereoselective_and_Environmentally_Friendly_Reactions
P. 216
192 8 Racemizable Acyl Donors for Enzymatic Dynamic Kinetic Resolution
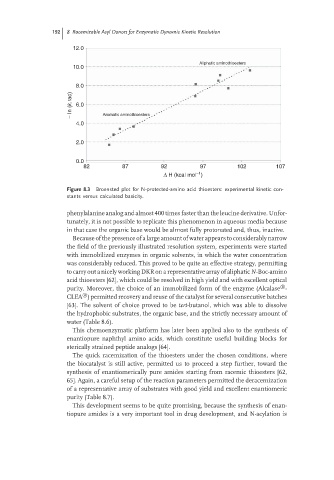
12.0
Aliphatic aminothioesters
10.0
8.0
− In (k.rac) 6.0
4.0 Aromatic aminothioesters
2.0
0.0
82 87 92 97 102 107
−1
Δ H (kcal mol )
Figure 8.3 Broensted plot for N-protected-amino acid thioesters: experimental kinetic con-
stants versus calculated basicity.
phenylalanine analog and almost 400 times faster than the leucine derivative. Unfor-
tunately, it is not possible to replicate this phenomenon in aqueous media because
in that case the organic base would be almost fully protonated and, thus, inactive.
Because of the presence of a large amount of water appears to considerably narrow
the field of the previously illustrated resolution system, experiments were started
with immobilized enzymes in organic solvents, in which the water concentration
was considerably reduced. This proved to be quite an effective strategy, permitting
to carry out a nicely working DKR on a representative array of aliphatic N-Boc-amino
acid thioesters [62], which could be resolved in high yield and with excellent optical
purity. Moreover, the choice of an immobilized form of the enzyme (Alcalase ® -
CLEA ® ) permitted recovery and reuse of the catalyst for several consecutive batches
[63]. The solvent of choice proved to be tert-butanol, which was able to dissolve
the hydrophobic substrates, the organic base, and the strictly necessary amount of
water (Table 8.6).
This chemoenzymatic platform has later been applied also to the synthesis of
enantiopure naphthyl amino acids, which constitute useful building blocks for
sterically strained peptide analogs [64].
The quick racemization of the thioesters under the chosen conditions, where
the biocatalyst is still active, permitted us to proceed a step further, toward the
synthesis of enantiomerically pure amides starting from racemic thioesters [62,
65]. Again, a careful setup of the reaction parameters permitted the deracemization
of a representative array of substrates with good yield and excellent enantiomeric
purity (Table 8.7).
This development seems to be quite promising, because the synthesis of enan-
tiopure amides is a very important tool in drug development, and N-acylation is