Page 217 - Cascade_Biocatalysis_Integrating_Stereoselective_and_Environmentally_Friendly_Reactions
P. 217
8.4 Conclusions 193
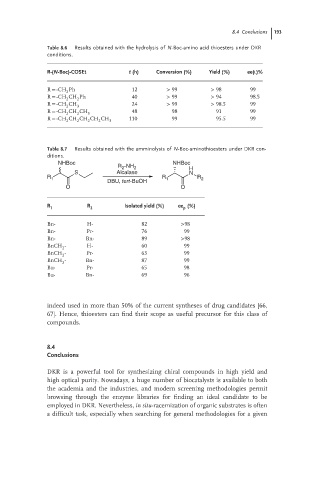
Table 8.6 Results obtained with the hydrolysis of N-Boc-amino acid thioesters under DKR
conditions.
R-(N-Boc)-COSEt t (h) Conversion (%) Yield (%) ee(L)%
R = -CH Ph 12 > 99 > 98 99
2
R = -CH CH Ph 40 > 99 > 94 98.5
2
2
R = -CH CH 24 > 99 > 98.5 99
2 3
R = -CH CH CH 3 48 98 91 99
2
2
R = -CH CH CH CH CH 110 99 95.5 99
2 2 2 2 3
Table 8.7 Results obtained with the amminolysis of N-Boc-aminothioesters under DKR con-
ditions.
NHBoc NHBoc
R 2 -NH 2 H
S Alcalase N
R 1 R 1 R 2
DBU, tert-BuOH
O O
R 1 R 2 Isolated yield (%) ee (%)
p
Bn- H- 82 >98
Bn- Pr- 76 99
Bn- Bn- 89 >98
BnCH - H- 60 99
2
BnCH - Pr- 63 99
2
BnCH - Bn- 87 99
2
Bu- Pr- 65 98
Bu- Bn- 69 96
indeed used in more than 50% of the current syntheses of drug candidates [66,
67]. Hence, thioesters can find their scope as useful precursor for this class of
compounds.
8.4
Conclusions
DKR is a powerful tool for synthesizing chiral compounds in high yield and
high optical purity. Nowadays, a huge number of biocatalysts is available to both
the academia and the industries, and modern screening methodologies permit
browsing through the enzyme libraries for finding an ideal candidate to be
employed in DKR. Nevertheless, in situ-racemization of organic substrates is often
a difficult task, especially when searching for general methodologies for a given