Page 212 - Cascade_Biocatalysis_Integrating_Stereoselective_and_Environmentally_Friendly_Reactions
P. 212
188 8 Racemizable Acyl Donors for Enzymatic Dynamic Kinetic Resolution
Table 8.4 Results obtained by deracemization of aminoesters by using picolinaldehyde/zinc
acetate mediated racemization in combination with enzymatic hydrolysis.
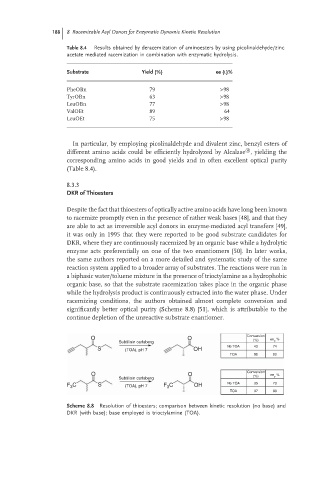
Substrate Yield (%) ee (L)%
PheOBn 79 >98
TyrOBn 63 >98
LeuOBn 77 >98
ValOEt 89 64
LeuOEt 75 >98
In particular, by employing picolinaldehyde and divalent zinc, benzyl esters of
different amino acids could be efficiently hydrolyzed by Alcalase ® , yielding the
corresponding amino acids in good yields and in often excellent optical purity
(Table 8.4).
8.3.3
DKR of Thioesters
Despite the fact that thioesters of optically active amino acids have long been known
to racemize promptly even in the presence of rather weak bases [48], and that they
are able to act as irreversible acyl donors in enzyme-mediated acyl transfers [49],
it was only in 1995 that they were reported to be good substrate candidates for
DKR, where they are continuously racemized by an organic base while a hydrolytic
enzyme acts preferentially on one of the two enantiomers [50]. In later works,
the same authors reported on a more detailed and systematic study of the same
reaction system applied to a broader array of substrates. The reactions were run in
a biphasic water/toluene mixture in the presence of trioctylamine as a hydrophobic
organic base, so that the substrate racemization takes place in the organic phase
while the hydrolysis product is continuously extracted into the water phase. Under
racemizing conditions, the authors obtained almost complete conversion and
significantly better optical purity (Scheme 8.8) [51], which is attributable to the
continue depletion of the unreactive substrate enantiomer.
O O Conversion ee p %
(%)
Subtilisin carlsberg
No TOA 43 74
S (TOA), pH 7 OH
TOA 95 80
O O Conversion ee p %
(%)
Subtilisin carlsberg
F 3 C S (TOA), pH 7 F 3 C OH No TOA 35 73
TOA 97 83
Scheme 8.8 Resolution of thioesters; comparison between kinetic resolution (no base) and
DKR (with base); base employed is trioctylamine (TOA).