Page 215 - Cascade_Biocatalysis_Integrating_Stereoselective_and_Environmentally_Friendly_Reactions
P. 215
8.3 Applications of DKR to Acyl Compounds 191
6.0
NHBoc NHBoc
SEt Alcalase, pH 8 OH
5.0
MTBE, (TOA) 100% conversion
O TOA O
vol NaOH 0.1 M (ml) 3.0 addition 50% conversion
4.0
2.0
1.0
0% conversion
0.0
0.0 10.0 20.0 30.0 40.0 50.0 60.0 70.0 80.0 90.0
Time (h)
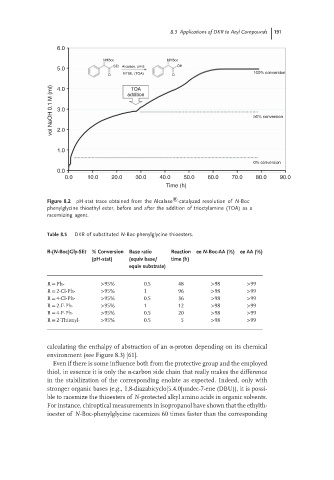
Figure 8.2 pH-stat trace obtained from the Alcalase ® -catalyzed resolution of N-Boc
phenylglycine thioethyl ester, before and after the addition of trioctylamine (TOA) as a
racemizing agent.
Table 8.5 DKR of substituted N-Boc-phenylglycine thioesters.
R-(N-Boc)Gly-SEt % Conversion Base ratio Reaction ee N-Boc-AA (%) ee AA (%)
(pH-stat) (equiv base/ time (h)
equiv substrate)
R = Ph- >95% 0.5 48 >98 >99
R = 2-Cl-Ph- >95% 1 96 >98 >99
R = 4-Cl-Ph- >95% 0.5 36 >98 >99
R = 2-F-Ph- >95% 1 12 >98 >99
R = 4-F-Ph- >95% 0.5 20 >98 >99
R = 2-Thienyl- >95% 0.5 5 >98 >99
calculating the enthalpy of abstraction of an α-proton depending on its chemical
environment (see Figure 8.3) [61].
Even if there is some influence both from the protective group and the employed
thiol, in essence it is only the α-carbon side chain that really makes the difference
in the stabilization of the corresponding enolate as expected. Indeed, only with
stronger organic bases (e.g., 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU)), it is possi-
ble to racemize the thioesters of N-protected alkyl amino acids in organic solvents.
For instance, chiroptical measurements in isopropanol have shown that the ethylth-
ioester of N-Boc-phenylglycine racemizes 60 times faster than the corresponding