Page 211 - Cascade_Biocatalysis_Integrating_Stereoselective_and_Environmentally_Friendly_Reactions
P. 211
8.3 Applications of DKR to Acyl Compounds 187
Table 8.3 DKR of aminoesters with Alcalase ® in the presence of 3,5-dinitrosalicylaldehyde.
Substrate Time (d) Yield (%) ee (%)
PheOEt 1 95 99 (L)
TyrOEt 1 87 97 (L)
MetOEt 2 96 89 (L)
ValOEt 4 98 64 (L)
LeuOEt 3 99 97 (L)
In order to increase the stereoselectivity and to minimize the competing hydroly-
sis, the reaction can be performed at low temperatures, where the enantioselectivity
parameter E is maximized.
Other aldehydes have been found to efficiently promote the epimerization of
the α-carbon. In 2008, Beller et al. [46] described an efficient deracemization of α-
aminoesters, where different aldehydes were screened to evaluate their respective
performance. In this study, 3,5-dinitrosalicylaldehyde proved to be particularly
efficient for the task, permitting to synthesize several amino acids in high yield and
often high enantiomeric excess (Table 8.3).
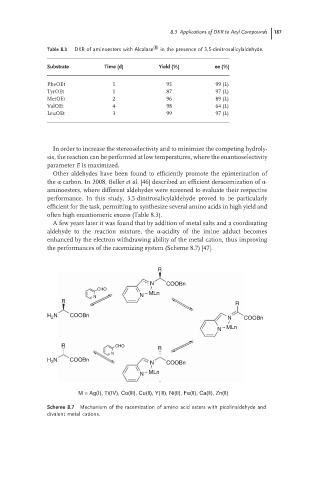
A few years later it was found that by addition of metal salts and a coordinating
aldehyde to the reaction mixture, the α-acidity of the imine adduct becomes
enhanced by the electron withdrawing ability of the metal cation, thus improving
the performances of the racemizing system (Scheme 8.7) [47].
R
N COOBn
CHO
MLn
N
N
R
R
H N COOBn N COOBn
2
N MLn
R CHO
R
N
H N COOBn N COOBn
2
N MLn
M = Ag(I), Ti(IV), Co(lll), Cu(ll), Y(lll), Ni(ll), Fe(ll), Ca(ll), Zn(ll)
Scheme 8.7 Mechanism of the racemization of amino acid esters with picolinaldehyde and
divalent metal cations.