Page 312 - Cascade_Biocatalysis_Integrating_Stereoselective_and_Environmentally_Friendly_Reactions
P. 312
288 13 Key-Study on NHase/AMase System
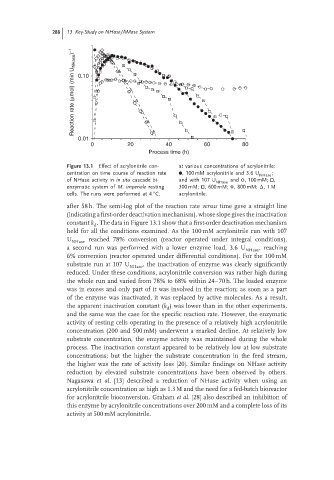
Reaction rate (μmol) (min U NHase ) −1 0.10
0.01
0 20 40 60 80
Process time (h)
Figure 13.1 Effect of acrylonitrile con- at various concentrations of acrylonitrile:
centration on time course of reaction rate , 100 mM acrylonitrile and 3.6 U ;
NHase
of NHase activity in in situ cascade bi- and with 107 U and ,100 mM; ,
NHase
enzymatic system of M. imperiale resting 300 mM; , 600 mM; , 800 mM; Δ,1 M
◦
cells. The runs were performed at 4 C, acrylonitrile.
after 58 h. The semi-log plot of the reaction rate versus time gave a straight line
(indicating a first-order deactivation mechanism), whose slope gives the inactivation
constant k . The data in Figure 13.1 show that a first-order deactivation mechanism
d
held for all the conditions examined. As the 100 mM acrylonitrile run with 107
U reached 78% conversion (reactor operated under integral conditions),
NHase
a second run was performed with a lower enzyme load, 3.6 U , reaching
NHase
6% conversion (reactor operated under differential conditions). For the 100 mM
substrate run at 107 U , the inactivation of enzyme was clearly significantly
NHase
reduced. Under these conditions, acrylonitrile conversion was rather high during
the whole run and varied from 78% to 68% within 24–70 h. The loaded enzyme
was in excess and only part of it was involved in the reaction; as soon as a part
of the enzyme was inactivated, it was replaced by active molecules. As a result,
the apparent inactivation constant (k ) was lower than in the other experiments,
d
and the same was the case for the specific reaction rate. However, the enzymatic
activity of resting cells operating in the presence of a relatively high acrylonitrile
concentration (200 and 500 mM) underwent a marked decline. At relatively low
substrate concentration, the enzyme activity was maintained during the whole
process. The inactivation constant appeared to be relatively low at low substrate
concentrations; but the higher the substrate concentration in the feed stream,
the higher was the rate of activity loss [20]. Similar findings on NHase activity
reduction by elevated substrate concentrations have been observed by others.
Nagasawa et al. [13] described a reduction of NHase activity when using an
acrylonitrile concentration as high as 1.3 M and the need for a fed-batch bioreactor
for acrylonitrile bioconversion. Graham et al. [28] also described an inhibition of
this enzyme by acrylonitrile concentrations over 200 mM and a complete loss of its
activity at 500 mM acrylonitrile.