Page 315 - Cascade_Biocatalysis_Integrating_Stereoselective_and_Environmentally_Friendly_Reactions
P. 315
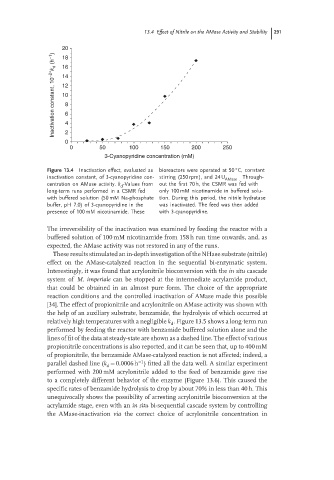
13.4 Effect of Nitrile on the AMase Activity and Stability 291
20
Inactivation constant, 10 −2∗ k d (h −1 ) 14 8 6
18
16
12
10
0 4 2
0 50 100 150 200 250
3-Cyanopyridine concentration (mM)
◦
Figure 13.4 Inactivation effect, evaluated as bioreactors were operated at 50 C, constant
inactivation constant, of 3-cyanopyridine con- stirring (250 rpm), and 24 U Through-
AMase .
centration on AMase activity. k -Values from out the first 70 h, the CSMR was fed with
d
long-term runs performed in a CSMR fed only 100 mM nicotinamide in buffered solu-
with buffered solution (50 mM Na-phosphate tion. During this period, the nitrile hydratase
buffer, pH 7.0) of 3-cyanopyridine in the was inactivated. The feed was then added
presence of 100 mM nicotinamide. These with 3-cyanopyridine.
The irreversibility of the inactivation was examined by feeding the reactor with a
buffered solution of 100 mM nicotinamide from 158 h run time onwards, and, as
expected, the AMase activity was not restored in any of the runs.
These results stimulated an in-depth investigation of the NHase substrate (nitrile)
effect on the AMase-catalyzed reaction in the sequential bi-enzymatic system.
Interestingly, it was found that acrylonitrile bioconversion with the in situ cascade
system of M. imperiale can be stopped at the intermediate acrylamide product,
that could be obtained in an almost pure form. The choice of the appropriate
reaction conditions and the controlled inactivation of AMase made this possible
[34]. The effect of propionitrile and acrylonitrile on AMase activity was shown with
the help of an auxiliary substrate, benzamide, the hydrolysis of which occurred at
relatively high temperatures with a negligible k . Figure 13.5 shows a long-term run
d
performed by feeding the reactor with benzamide buffered solution alone and the
lines of fit of the data at steady-state are shown as a dashed line. The effect of various
propionitrile concentrations is also reported, and it can be seen that, up to 400 mM
of propionitrile, the benzamide AMase-catalyzed reaction is not affected; indeed, a
−1
parallel dashed line (k = 0.0006 h ) fitted all the data well. A similar experiment
d
performed with 200 mM acrylonitrile added to the feed of benzamide gave rise
to a completely different behavior of the enzyme (Figure 13.6). This caused the
specific rates of benzamide hydrolysis to drop by about 70% in less than 40 h. This
unequivocally shows the possibility of arresting acrylonitrile bioconversion at the
acrylamide stage, even with an in situ bi-sequential cascade system by controlling
the AMase-inactivation via the correct choice of acrylonitrile concentration in