Page 314 - Cascade_Biocatalysis_Integrating_Stereoselective_and_Environmentally_Friendly_Reactions
P. 314
290 13 Key-Study on NHase/AMase System
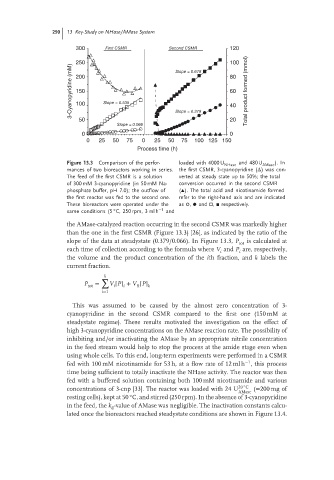
300 First CSMR Second CSMR 120
250 Slope = 0.618 100
3-Cyanopyridine (mM) 150 Slope = 0.535 60 Total product formed (mmol)
200
80
100
40
50 Slope = 0.379 20
Slope = 0.066
0 0
0 25 50 75 0 25 50 75 100 125 150
Process time (h)
Figure 13.3 Comparison of the perfor- loaded with 4000 U NHase and 480 U AMase ). In
mances of two bioreactors working in series. the first CSMR, 3-cyanopyridine (Δ) was con-
The feed of the first CSMR is a solution verted at steady state up to 50%; the total
of 300 mM 3-cyanopyridine (in 50 mM Na- conversion occurred in the second CSMR
phosphate buffer, pH 7.0); the outflow of (▴). The total acid and nicotinamide formed
the first reactor was fed to the second one. refer to the right-hand axis and are indicated
These bioreactors were operated under the as , and , respectively.
◦
same conditions (5 C, 250 rpm, 3 ml h −1 and
the AMase-catalyzed reaction occurring in the second CSMR was markedly higher
than the one in the first CSMR (Figure 13.3) [26], as indicated by the ratio of the
slope of the data at steadystate (0.379/0.066). In Figure 13.3, P tot is calculated at
each time of collection according to the formula where V and P are, respectively,
i
i
the volume and the product concentration of the ith fraction, and k labels the
current fraction.
k
∑
P tot = V [P] + V [P] k
i
i
R
i=1
This was assumed to be caused by the almost zero concentration of 3-
cyanopyridine in the second CSMR compared to the first one (150 mM at
steadystate regime). These results motivated the investigation on the effect of
high 3-cyanopyridine concentrations on the AMase reaction rate. The possibility of
inhibiting and/or inactivating the AMase by an appropriate nitrile concentration
in the feed stream would help to stop the process at the amide stage even when
using whole cells. To this end, long-term experiments were performed in a CSMR
−1
fed with 100 mM nicotinamide for 53 h, at a flow rate of 12 ml h ,thisprocess
time being sufficient to totally inactivate the NHase activity. The reactor was then
fed with a buffered solution containing both 100 mM nicotinamide and various
◦
concentrations of 3-cnp [33]. The reactor was loaded with 24 U 20 C (=200 mg of
AMase
◦
resting cells), kept at 50 C, and stirred (250 rpm). In the absence of 3-cyanopyridine
in the feed, the k -value of AMase was negligible. The inactivation constants calcu-
d
lated once the bioreactors reached steadystate conditions are shown in Figure 13.4.