Page 327 - Cascade_Biocatalysis_Integrating_Stereoselective_and_Environmentally_Friendly_Reactions
P. 327
14.3 Nitrile Hydrolysis Enzymes 303
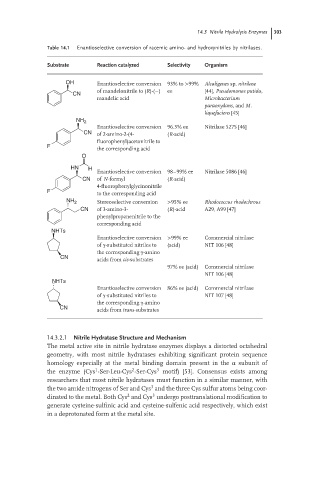
Table 14.1 Enantioselective conversion of racemic amino- and hydroxynitriles by nitrilases.
Substrate Reaction catalyzed Selectivity Organism
OH Enantioselective conversion 93% to >99% Alcaligenes sp. nitrilase
of mandelonitrile to (R)-(−) ee [44], Pseudomonas putida,
CN
mandelic acid Microbacterium
paraoxydans,and M.
liquefaciens [45]
NH 2
Enantioselective conversion 96.3% ee Nitrilase 5275 [46]
CN of 2-amino-2-(4- (R-acid)
fluorophenyl)acetonitrile to
F
the corresponding acid
O
HN H
Enantioselective conversion 98–99% ee Nitrilase 5086 [46]
CN of N-formyl (R-acid)
4-fluorophenylglycinonitrile
F to the corresponding acid
NH 2 Stereoselective conversion >95% ee Rhodococcus rhodochrous
CN of 3-amino-3- (R)-acid A29, A99 [47]
phenylpropanenitrile to the
corresponding acid
NHTs
Enantioselective conversion >99% ee Commercial nitrilase
of γ-substituted nitriles to (acid) NIT 106 [48]
the corresponding γ-amino
CN acids from cis-substrates
97% ee (acid) Commercial nitrilase
NIT 106 [48]
NHTs
Enantioselective conversion 86% ee (acid) Commercial nitrilase
of γ-substituted nitriles to NIT 107 [48]
the corresponding γ-amino
CN
acids from trans-substrates
14.3.2.1 Nitrile Hydratase Structure and Mechanism
The metal active site in nitrile hydratase enzymes displays a distorted octahedral
geometry, with most nitrile hydratases exhibiting significant protein sequence
homology especially at the metal binding domain present in the α subunit of
2
3
1
theenzyme(Cys -Ser-Leu-Cys -Ser-Cys motif) [53]. Consensus exists among
researchers that most nitrile hydratases must function in a similar manner, with
3
the two amide nitrogens of Ser and Cys and the three Cys sulfur atoms being coor-
2
3
dinated to the metal. Both Cys and Cys undergo posttranslational modification to
generate cysteine-sulfinic acid and cysteine-sulfenic acid respectively, which exist
in a deprotonated form at the metal site.