Page 322 - Cascade_Biocatalysis_Integrating_Stereoselective_and_Environmentally_Friendly_Reactions
P. 322
298 14 Enzymatic Stereoselective Synthesis of -Amino Acids
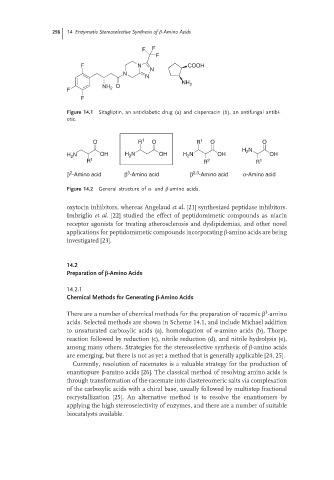
F F
F
F N COOH
N
N
N
NH
O 2
NH 2
F
F
Figure 14.1 Sitagliptin, an antidiabetic drug (a) and cispentacin (b), an antifungal antibi-
otic.
O R 1 O R 1 O O
H N
2
H N OH H 2 N OH H 2 N OH OH
2
R 1 R 2 R 1
2
3
β -Amino acid β -Amino acid β 2,3 -Amino acid α-Amino acid
Figure 14.2 General structure of α-and β-amino acids.
oxytocin inhibitors, whereas Angelaud et al. [21] synthesized peptidase inhibitors.
Imbriglio et al. [22] studied the effect of peptidomimetic compounds as niacin
receptor agonists for treating atherosclerosis and dyslipidemias, and other novel
applications for peptidomimetic compounds incorporating β-amino acids are being
investigated [23].
14.2
Preparation of -Amino Acids
14.2.1
Chemical Methods for Generating -Amino Acids
3
There are a number of chemical methods for the preparation of racemic β -amino
acids. Selected methods are shown in Scheme 14.1, and include Michael addition
to unsaturated carboxylic acids (a), homologation of α-amino acids (b), Thorpe
reaction followed by reduction (c), nitrile reduction (d), and nitrile hydrolysis (e),
among many others. Strategies for the stereoselective synthesis of β-amino acids
are emerging, but there is not as yet a method that is generally applicable [24, 25].
Currently, resolution of racemates is a valuable strategy for the production of
enantiopure β-amino acids [26]. The classical method of resolving amino acids is
through transformation of the racemate into diastereomeric salts via complexation
of the carboxylic acids with a chiral base, usually followed by multistep fractional
recrystallization [25]. An alternative method is to resolve the enantiomers by
applying the high stereoselectivity of enzymes, and there are a number of suitable
biocatalysts available.