Page 323 - Cascade_Biocatalysis_Integrating_Stereoselective_and_Environmentally_Friendly_Reactions
P. 323
14.2 Preparation of β-Amino Acids 299
NH 2 NH 2
CO 2 H
R 2 + a R 2 CO H e R 2 CN
2
R 1 RNH 2 1
R R 1
b d
c
NH 2 NC CO 2 R
+ CH N
2 2
R 2 COOH R 1
2
R CN + CH 3 CN
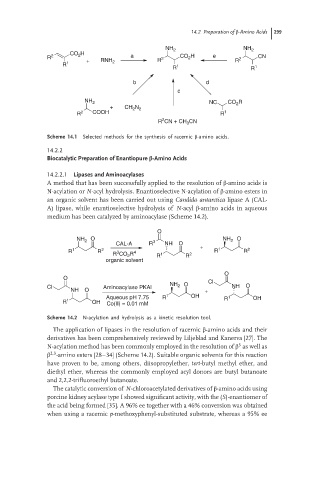
Scheme 14.1 Selected methods for the synthesis of racemic β-amino acids.
14.2.2
Biocatalytic Preparation of Enantiopure -Amino Acids
14.2.2.1 Lipases and Aminoacylases
A method that has been successfully applied to the resolution of β-amino acids is
N-acylation or N-acyl hydrolysis. Enantioselective N-acylation of β-amino esters in
an organic solvent has been carried out using Candida antarctica lipase A (CAL-
A) lipase, while enantioselective hydrolysis of N-acyl β-amino acids in aqueous
medium has been catalyzed by aminoacylase (Scheme 14.2).
O
O O
NH 2 3 NH 2
CAL-A R NH O
+
R 1 R 2 3 4 R 1 R 2
R CO R R 1 R 2
2
organic solvent
O
O
NH O Cl
Cl Aminoacylase PKAI 2 NH O
NH O +
Aqueous pH 7.75 R 1 OH R 1 OH
R 1 OH Co(II) = 0.01 mM
Scheme 14.2 N-acylation and hydrolysis as a kinetic resolution tool.
The application of lipases in the resolution of racemic β-amino acids and their
derivatives has been comprehensively reviewed by Liljeblad and Kanerva [27]. The
3
N-acylation method has been commonly employed in the resolution of β as well as
2,3
β -amino esters [28–34] (Scheme 14.2). Suitable organic solvents for this reaction
have proven to be, among others, diisopropylether, tert-butyl methyl ether, and
diethyl ether, whereas the commonly employed acyl donors are butyl butanoate
and 2,2,2-trifluoroethyl butanoate.
The catalytic conversion of N-chloroacetylated derivatives of β-amino acids using
porcine kidney acylase type I showed significant activity, with the (S)-enantiomer of
the acid being formed [35]. A 96% ee together with a 46% conversion was obtained
when using a racemic p-methoxyphenyl-substituted substrate, whereas a 95% ee