Page 378 - Cascade_Biocatalysis_Integrating_Stereoselective_and_Environmentally_Friendly_Reactions
P. 378
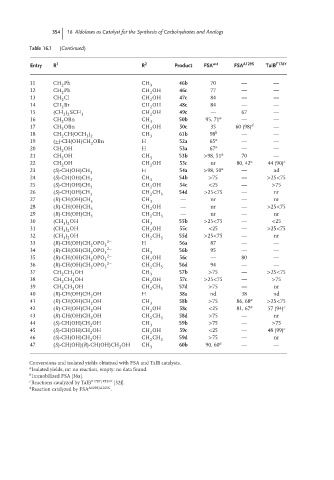
354 16 Aldolases as Catalyst for the Synthesis of Carbohydrates and Analogs
Table 16.1 (Continued)
Entry R 1 R 2 Product FSA w-t FSA A129S TalB F178Y
11 CH Ph CH 46b 70 — —
2 3
12 CH Ph CH OH 46c 77 — —
2
2
13 CH Cl CH OH 47c 84 — —
2
2
14 CH Br CH OH 48c 84 — —
2 2
15 (CH ) SCH 3 CH OH 49c — 67 —
2
2 2
16 CH OBn CH 50b 95, 71 a — —
2 3
17 CH OBn CH OH 50c 35 60 (98) d —
2
2
18 CH CH(OCH ) CH 61b 98 b — —
2 3 2 3
19 (±)-CH(OH)CH OBn H 52a 65 a — —
2
20 CH OH H 53a 67 a — —
2
21 CH OH CH 3 53b >98, 51 a 70 —
2
22 CH OH CH OH 53c nr 80, 42 a 44 (90) c
2 2
23 (S)-CH(OH)CH 3 H 54a >98, 50 a — nd
24 (S)-CH(OH)CH CH 54b >75 — >25<75
3 3
25 (S)-CH(OH)CH CH OH 54c <25 — >75
3 2
26 (S)-CH(OH)CH 3 CH CH 3 54d >25<75 — nr
2
27 (R)-CH(OH)CH CH — nr — nr
3 3
28 (R)-CH(OH)CH 3 CH OH — nr — >25<75
2
29 (R)-CH(OH)CH CH CH — nr — nr
3 2 3
30 (CH ) OH CH 3 55b >25<75 — <25
3 2
31 (CH ) OH CH OH 55c <25 — >25<75
3 2 2
32 (CH ) OH CH CH 3 55d >25<75 — nr
3 2
2
33 (R)-CH(OH)CH OPO 2− H 56a 87 — —
2 3
34 (R)-CH(OH)CH OPO 3 2− CH 3 56b 95 — —
2
35 (R)-CH(OH)CH OPO 2− CH OH 56c — 80 —
2 3 2
36 (R)-CH(OH)CH OPO 3 2− CH CH 3 56d 94 — —
2
2
37 CH CH OH CH 57b >75 — >25<75
2 2 3
38 CH CH OH CH OH 57c >25<75 — >75
2 2 2
39 CH CH OH CH CH 3 57d >75 — nr
2
2
2
40 (R)-CH(OH)CH OH H 58a nd 38 nd
2
41 (R)-CH(OH)CH OH CH 3 58b >75 86, 68 a >25<75
2
42 (R)-CH(OH)CH OH CH OH 58c <25 81, 67 a 57 (94) c
2 2
43 (R)-CH(OH)CH OH CH CH 3 58d >75 — nr
2
2
44 (S)-CH(OH)CH OH CH 59b >75 — >75
2 3
45 (S)-CH(OH)CH OH CH OH 59c <25 — 48 (99) c
2
2
46 (S)-CH(OH)CH OH CH CH 59d >75 — nr
2 2 3
47 (S)-CH(OH)(R)-CH(OH)CH OH CH 3 60b 90, 60 a — —
2
Conversions and isolated yields obtained with FSA and TalB catalysts.
a
Isolated yields, nr: no reaction, empty: no data found.
b Immobilized FSA [36a].
c F178Y/R181E
Reactions catalyzed by TalB [32f].
d Reaction catalyzed by FSA A129S/A165G .