Page 374 - Cascade_Biocatalysis_Integrating_Stereoselective_and_Environmentally_Friendly_Reactions
P. 374
350 16 Aldolases as Catalyst for the Synthesis of Carbohydrates and Analogs
However, the use of FSA as catalyst faces some problems such as low tolerance
toward α-substituted aldehyde donors and tolerance toward the acceptor depending
on the quality of the donor substrate. In an attempt to overcome this limitation,
we envisaged a program of site-directed mutagenesis of key amino acids in the
active site of FSA oriented to improve the tolerance toward N-Cbz-amino aldehydes
for the preparation of iminocyclitols [34]. In the first mutagenic round, FSA A165G
made possible the reaction with α-substituted N-Cbz-aminoaldehydes (S)-, (R)-24j,l
(Scheme 16.7). Interestingly, FSA A129S/A165G resulted in a high synergistic double
mutation for reactions with both HA and DHA donors, with an activity between
5- and >900-fold higher than that of wild type toward N-Cbz-aminoaldehyde
derivatives 24a,b, h–m (Scheme 16.7) [34]. It had been previously found that
mutant FSA A129S had an improved activity toward DHA as donor (see below) [32e,
35]. Protein molecular models confirmed that the A165G mutation generates the
required space to allocate the C-α methyl group of (S)- and (R)-24j,l without clashing
with the protein residues, and suggested a potentially activating polar interaction
of residue S129 with the nucleophile enamine–enzyme intermediate [34]. Further
works to ascertain the substrate scope of FSA A129S/A165G and other mutations in the
acceptor binding site are currently in progress in our group.
Previous works show that the FSA A129S mutant, designed to resemble the donor
binding site of TalB [35], exhibited improved tolerance toward DHA and resulted in
an efficient tool in the preparation of carbohydrates and analogs [32e]. FSA A129S was
shown to be a good catalyst for the synthesis of aminocyclitols prepared by a two-
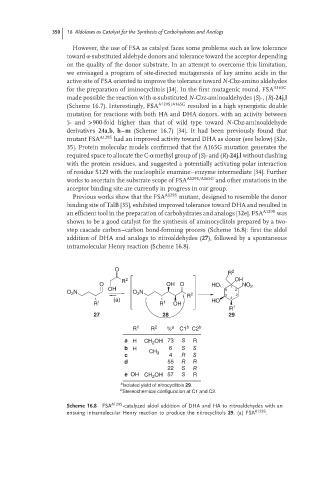
step cascade carbon–carbon bond-forming process (Scheme 16.8): first the aldol
addition of DHA and analogs to nitroaldehydes (27), followed by a spontaneous
intramolecular Henry reaction (Scheme 16.8).
O
R 2
R 2 OH
O OH O HO NO 2
OH 6 1 2
O 2 N O 2 N
R 2 5 3
(a) HO 4
R 1 R 1 OH
R 1
27 28 29
b
R 1 R 2 % a C1 C2 b
a H CH 2 OH 73 S R
b H 6 S S
CH 3
c 4 R S
d 55 R R
22 S R
e OH CH 2 OH 57 S R
a
Isolated yield of nitrocyclitols 29.
b
Stereochemical configuration at C1 and C2.
Scheme 16.8 FSA A129S -catalyzed aldol addition of DHA and HA to nitroaldehydes with an
ensuing intramolecular Henry reaction to produce the nitrocyclitols 29.(a) FSA A129S .