Page 373 - Cascade_Biocatalysis_Integrating_Stereoselective_and_Environmentally_Friendly_Reactions
P. 373
16.2 Iminocyclitol and Aminocyclitol Synthesis 349
of biocatalytic aldol additions. In addition to DHA, both enzymes also accept
structural analogs of DHA such as glycolaldehyde (GO), hydroxyacetone (HA), and
hydroxybutanone (HB) as donor substrates, as well as structurally diverse acceptor
aldehydes [5c, 24, 32]. This was a first step to overcome one of the limitations
of DHAP aldolases, namely their strict specificity toward the donor substrate.
Moreover, FSA and RhuA, both using unphosphorylated DHA as donor, furnish
stereocomplementary aldol adducts that are highly valuable for the development of
biocatalytic aldol additions.
We reported the first example of the use of FSA in iminocyclitol synthesis:
the preparation of d-fagomine, a piperidine-type iminocyclitol first isolated from
buckwheat seeds of Fagopyrum esculentum Moench [32b, 33]. Following this work, a
number of related analog compounds of d-fagomine- and pyrrolidine-type iminocy-
clitols from aldol additions of HA and HB to N-Cbz-3-aminopropanal and azido
aldehydes using FSA as catalyst have been reported by Wong and coworkers [32c].
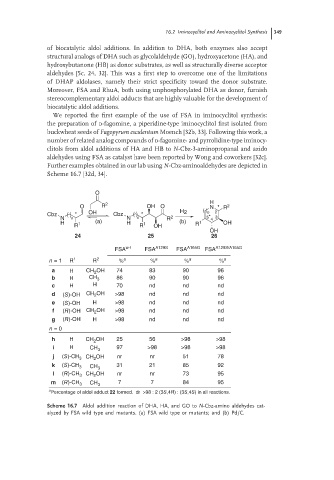
Further examples obtained in our lab using N-Cbz-aminoaldehydes are depicted in
Scheme 16.7 [32d, 34].
O
O R 2 OH O H * * R 2
N
Cbz * * OH Cbz * * H 2 n 2
N n N n R 2 5* 4 3
H R 1 (a) H R 1 OH (b) R 1 OH
OH
24 25 26
FSA w-t FSA A129S FSA A165G FSA A129S/A165G
n = 1 R 1 R 2 % a % a % a % a
a H CH OH 74 83 90 96
2
b H CH 3 86 90 90 96
c H H 70 nd nd nd
d (S)-OH CH 2 OH >98 nd nd nd
e (S)-OH H >98 nd nd nd
f (R)-OH CH 2 OH >98 nd nd nd
g (R)-OH H >98 nd nd nd
n = 0
h H CH OH 25 56 >98 >98
2
i H CH 3 97 >98 >98 >98
j (S)-CH 3 CH 2 OH nr nr 51 78
k (S)-CH 3 CH 3 31 21 85 92
l (R)-CH 3 CH OH nr nr 73 95
2
m (R)-CH 3 CH 3 7 7 84 95
a
Percentage of aldol adduct 22 formed. dr >98 : 2 (3S,4R) : (3S,4S) in all reactions.
Scheme 16.7 Aldol addition reaction of DHA, HA, and GO to N-Cbz-amino aldehydes cat-
alyzed by FSA wild type and mutants. (a) FSA wild type or mutants; and (b) Pd/C.