Page 368 - Cascade_Biocatalysis_Integrating_Stereoselective_and_Environmentally_Friendly_Reactions
P. 368
344 16 Aldolases as Catalyst for the Synthesis of Carbohydrates and Analogs
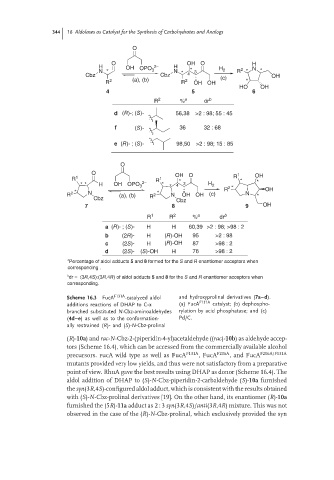
O
O OH O H
H 2− H
OH OPO 3 * H 2 2 N
N * * N * 3 R * *
Cbz Cbz 4 (c) OH
R 2 (a), (b) R 2 OH OH *
HO OH
4 5 6
R 2 % a dr b
d (R)-; (S)- 56,38 >2 : 98; 55 : 45
f (S)- 36 32 : 68
e (R)- ; (S)- 98,50 >2 : 98; 15 : 85
O
O OH O 1 OH
R 1 R 1 * R *
* * H OH OPO 3 2− * * 4 3 H 2 R 2 * * OH
R 2 * N (a), (b) R 2 * N OH OH (c) N *
Cbz Cbz
7 8 9 OH
R 1 R 2 % a dr b
a (R)- ; (S)- H H 60,39 >2 : 98; >98 : 2
b (2R)- H (R)-OH 95 >2 : 98
c (2S)- H (R)-OH 87 >98 : 2
d (2S)- (S)-OH H 76 >98 : 2
Percentage of aldol adducts 5 and 8 formed for the S and R enantiomer acceptors when
a
corresponding .
b
dr = (3R,4S):(3R,4R) of aldol adducts 5 and 8 for the S and R enantiomer acceptors when
corresponding.
Scheme 16.3 FucA F131A -catalyzed aldol and hydroxyprolinal derivatives (7a–d).
additions reactions of DHAP to C-α (a) FucA F131A catalyst; (b) dephospho-
branched substituted N-Cbz-aminoaldehydes rylation by acid phosphatase; and (c)
(4d–e) as well as to the conformation- Pd/C.
ally restrained (R)- and (S)-N-Cbz-prolinal
(R)-10a)and rac-N-Cbz-2-(piperidin-4-yl)acetaldehyde ((rac)-10b) as aldehyde accep-
tors (Scheme 16.4), which can be accessed from the commercially available alcohol
precursors. fucAwildtypeaswellasFucA F131A , FucA F206A , and FucA F206A/F131A
mutants provided very low yields, and thus were not satisfactory from a preparative
point of view. RhuA gave the best results using DHAP as donor (Scheme 16.4). The
aldol addition of DHAP to (S)-N-Cbz-piperidin-2-carbaldehyde (S)-10a furnished
the syn(3R,4S)-configured aldol adduct, which is consistent with the results obtained
with (S)-N-Cbz-prolinal derivatives [19]. On the other hand, its enantiomer (R)-10a
furnished the (5R)-11a adduct as 2 : 3 syn(3R,4S)/anti(3R,4R) mixture. This was not
observed in the case of the (R)-N-Cbz-prolinal, which exclusively provided the syn