Page 369 - Cascade_Biocatalysis_Integrating_Stereoselective_and_Environmentally_Friendly_Reactions
P. 369
16.2 Iminocyclitol and Aminocyclitol Synthesis 345
O
H
O OH O * OH
* OH OPO 3 2− 5 or 6 * * H 2 * n
N n N 4 3 N *
(a),(b) n (c) OH
Cbz Cbz OH OH
(S)-10a: n = 0 11a: n = 0 12: n = 0 OH
(R)-10a: n = 0 11b: n = 1 13: n = 1
(rac)-10b: n = 1
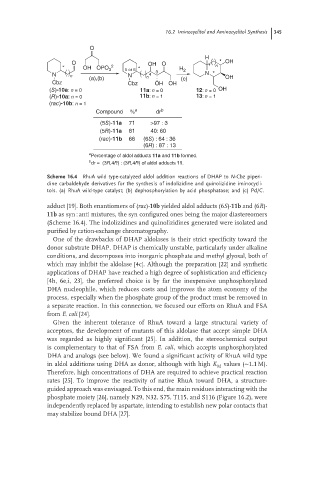
Compound % a dr b
(5S)-11a 71 >97 : 3
(5R)-11a 81 40: 60
(rac)-11b 66 (6S) : 64 : 36
(6R) : 87 : 13
a
Percentage of aldol adducts 11a and 11b formed.
b
dr = (3R,4R) : (3R,4R) of aldol adducts 11.
Scheme 16.4 RhuA wild type-catalyzed aldol addition reactions of DHAP to N-Cbz piperi-
dine carbaldehyde derivatives for the synthesis of indolizidine and quinolizidine iminocycli-
tols. (a) RhuA wild-type catalyst; (b) dephosphorylation by acid phosphatase; and (c) Pd/C.
adduct [19]. Both enantiomers of (rac)-10b yielded aldol adducts (6S)-11b and (6R)-
11b as syn : anti mixtures, the syn configured ones being the major diastereomers
(Scheme 16.4). The indolizidines and quinolizidines generated were isolated and
purified by cation-exchange chromatography.
One of the drawbacks of DHAP aldolases is their strict specificity toward the
donor substrate DHAP. DHAP is chemically unstable, particularly under alkaline
conditions, and decomposes into inorganic phosphate and methyl glyoxal, both of
which may inhibit the aldolase [4c]. Although the preparation [22] and synthetic
applications of DHAP have reached a high degree of sophistication and efficiency
[4h, 6e,i, 23], the preferred choice is by far the inexpensive unphosphorylated
DHA nucleophile, which reduces costs and improves the atom economy of the
process, especially when the phosphate group of the product must be removed in
a separate reaction. In this connection, we focused our efforts on RhuA and FSA
from E. coli [24].
Given the inherent tolerance of RhuA toward a large structural variety of
acceptors, the development of mutants of this aldolase that accept simple DHA
was regarded as highly significant [25]. In addition, the stereochemical output
is complementary to that of FSA from E. coli, which accepts unphosphorylated
DHA and analogs (see below). We found a significant activity of RhuA wild type
in aldol additions using DHA as donor, although with high K M values (∼1.1 M).
Therefore, high concentrations of DHA are required to achieve practical reaction
rates [25]. To improve the reactivity of native RhuA toward DHA, a structure-
guided approach was envisaged. To this end, the main residues interacting with the
phosphate moiety [26], namely N29, N32, S75, T115, and S116 (Figure 16.2), were
independently replaced by aspartate, intending to establish new polar contacts that
may stabilize bound DHA [27].